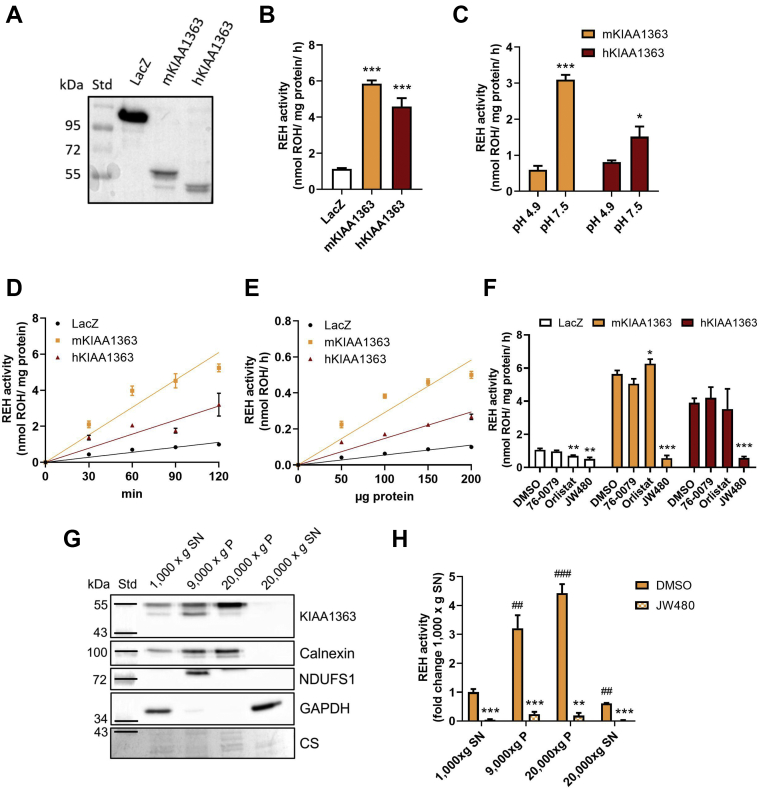
Fig. 1.
KIAA1363 exhibits RE hydrolase activity at neutral pH. Expi293F™ cells were transfected with plasmids encoding N-terminal HIS-tagged mKIAA1363 and hKIAA1363 or LacZ as control. Subsequently, lysates were prepared. A: Expression of HIS-tagged proteins was analyzed by Western blotting. B–F and H: For in vitro REH activity assay, cell lysates containing recombinant proteins were incubated with RP (300 μM) as substrate for 1 h. Substrate was emulsified with PC (300 μM) in potassium phosphate buffer (100 mM, pH 7.5) or potassium acetate buffer (100 mM, pH 4.9), as indicated, and 2% FA-free BSA were added. Retinoids were n-hexane extracted, and ROH content was analyzed by HPLC-FD. D: Cell lysates were incubated with substrate for various time points as indicated. E: Increasing amounts of cell lysates, as indicated, were incubated with substrate. F: Small-molecule inhibitors or DMSO as solvent control were added to the cell lysates: 76-0079 (20 μM), Orlistat (20 μM), or JW480 (10 μM). G and H: Cell lysates containing mKIAA1363 were fractionated by differential centrifugation: 1,000 g supernatant (SN), 9,000 g pellet (P, mitochondria-enriched/microsome-enriched fraction), 20,000 g P (microsome-enriched fraction), and 20,000 g SN (cytoplasmic-enriched fraction). G: Protein content of mKIAA1363, calnexin, GAPDH, and NDUFS1 was analyzed by Western blotting. Coomassie stain (CS) was used as loading control. H: Cell fractions were incubated with substrate containing DMSO (solvent control) or the small-molecule inhibitor, JW480 (10 μM). Data are mean + SD and representative for three independent experiments (n = 3). Statistically significant differences were determined by Student's unpaired t-test (two-tailed: ∗P < 0.01; ∗∗P < 0.01; ∗∗∗P < 0.001 between groups; ##P < 0.01; ###P < 0.001 between cell fractions).