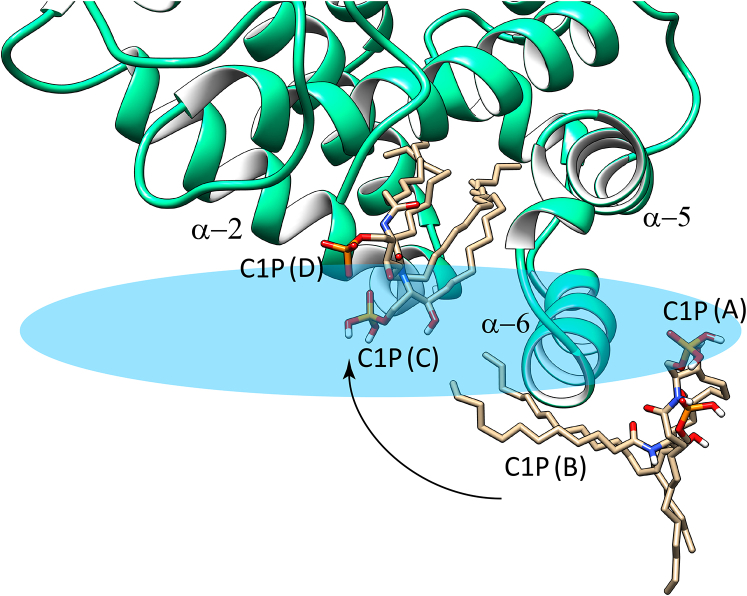
Fig. 8.
A model illustrating the functional role of α-6 helix for reorienting and guiding C1P into the binding pocket of CPTP from membrane. The progression of events that lead to C1P binding is shown by four different C1P conformations. C1P (A) represents C1P positioned normally with the POPC bilayer; C1P (B) represents C1P that has become reoriented and repositioned by interaction with α-6 helix where the C1P hydrocarbon chains interact with Trp152 and Val153, whereas the CIP phosphate interacts with di-Arg motif (R155 and R156). The reoriented C1P becomes much more aligned with the bilayer interface thus enabling access to the hydrophobic binding pocket of CPTP. C1P (C) is shown sliding into the binding pocket of CPTP. C1P (D) represents C1P secured within the hydrophobic pocket due to the specific interactions of the C1P polar headgroup, that is, phosphate and acyl-amide linkage, with residues that define the C1P recognition motif as previously identified by X-ray crystallography and mutation analyses.