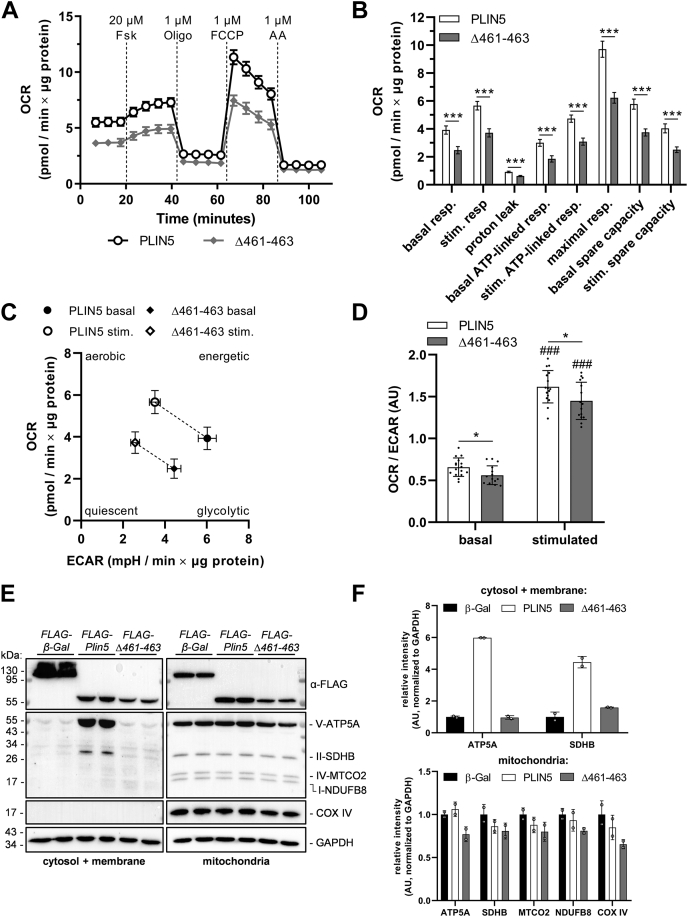
Fig. 8.
PLIN5-mediated LDMC augments the mitochondrial respiratory capacity and metabolic flexibility of lipid-challenged cardiomyocytes compared with cells overexpressing PLIN5(Δ461–463). AC16 cells stably overexpressing PLIN5 or PLIN5(Δ461–463) were cultured in the presence of 0.4 mM OA-BSA for 24 h, followed by respirometry analysis using a Seahorse XFe96 analyzer. Assay medium consisted of DMEM (catalog no.: D5030; Gibco) containing 5 mM glucose and 2 mM GlutaMAX, adjusted to pH 7.4 prior to measurement. A: OCR profiles upon sequential injection of forskolin (Fsk), oligomycin A (Oligo), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and antimycin A (AA), as indicated (n = 15–16). B: As measured in (A), quantification of basal, forskolin-stimulated (stim.), and maximal respiration (resp.), mitochondrial proton leak, as well as ATP-linked respiration and mitochondrial spare capacity under basal or stimulated conditions. C, D: Metabolic shift upon β-adrenergic stimulation was (C) visualized by plotting OCR against extracellular acidification rate (ECAR) and (D) quantified by calculating the OCR to ECAR ratio. E: Stable AC16 cells were treated with 0.4 mM OA-BSA for 24 h, followed by subcellular fractionation. Expression levels of ETC proteins, as indicated, in cytosol/membrane fractions or mitochondria-enriched fractions were determined by immunoblot analyses using an anti-total-OXPHOS-human antibody cocktail and an anti-COX IV antibody. GAPDH was probed as a marker for cytosolic crosscontamination and as loading control. F: Quantification of immunoblot signals (E) normalized to GAPDH. Data are presented as mean ± 95% CI (A, B) or mean ± SD (C, D). Statistical significance was determined by unpaired Student’s t-test (ns = not significant, ∗P < 0.05, ∗∗∗P < 0.001; ###P < 0.001 vs. corresponding basal conditions). AU, arbitrary unit.