Figure 1. Generation and specificity of novel monoclonal antibodies against human BAFF-R.
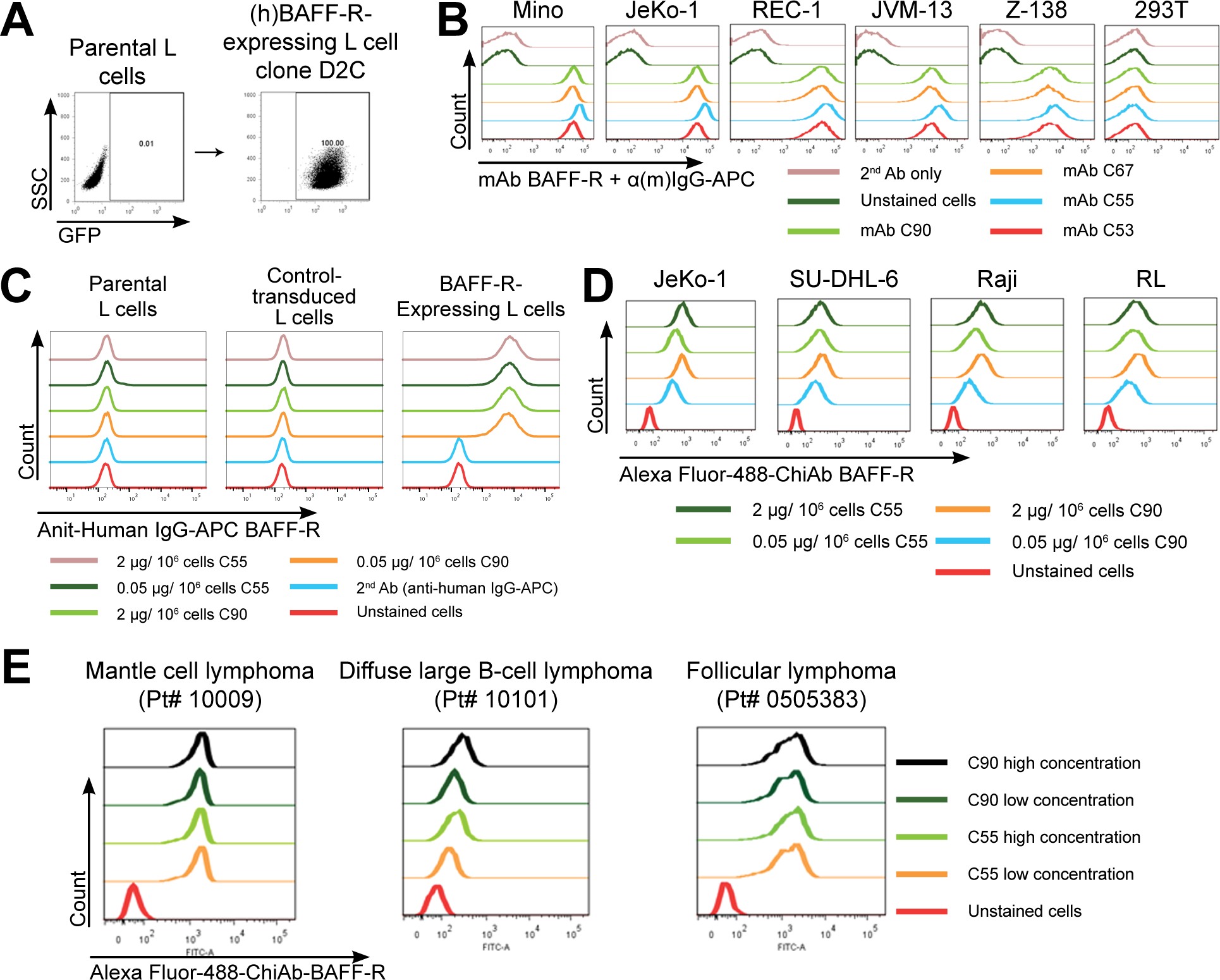
(A) FACS analysis of cell surface expression of (h)BAFF-R-GFP fusion protein in mouse fibroblast L cells. Gated on GFP-positive cells, engineered L cell clone (right plot) is compared to parental L cells (left plot). Clone D2C was selected for further studies. (B-E) FACS histograms of anti-BAFF-R monoclonal antibodies binding cell lines and patient samples: (B) Affinity purified hybridoma mAb (C90, C67, C55, and C53) binding BAFF-R-positive, human MCL lines including Mino, JeKo-1, REC-1, JVM-13, and Z-138 at a concentration of 0.05 μg mAb/106 cells. BAFF-R-negative 293T embryonic kidney cell line was used as a control. (C) Chimeric antibodies C55 and C90 at high and low concentration binding (h)BAFF-R-expressing L cells. Parental L cells, control-transduced L cells, and secondary anti-(h)IgG-APC antibodies only were used as controls. (D) Alexa fluor 488-conjugated chimeric antibodies binding a panel of NHL cell lines. (E) Chimeric antibodies binding three types of NHL primary patient samples. The data are representative of three independent experiments.
