Figure 2. BAFF-R monoclonal antibodies exhibited specific in vitro cytotoxicity against B-cell tumor lines.
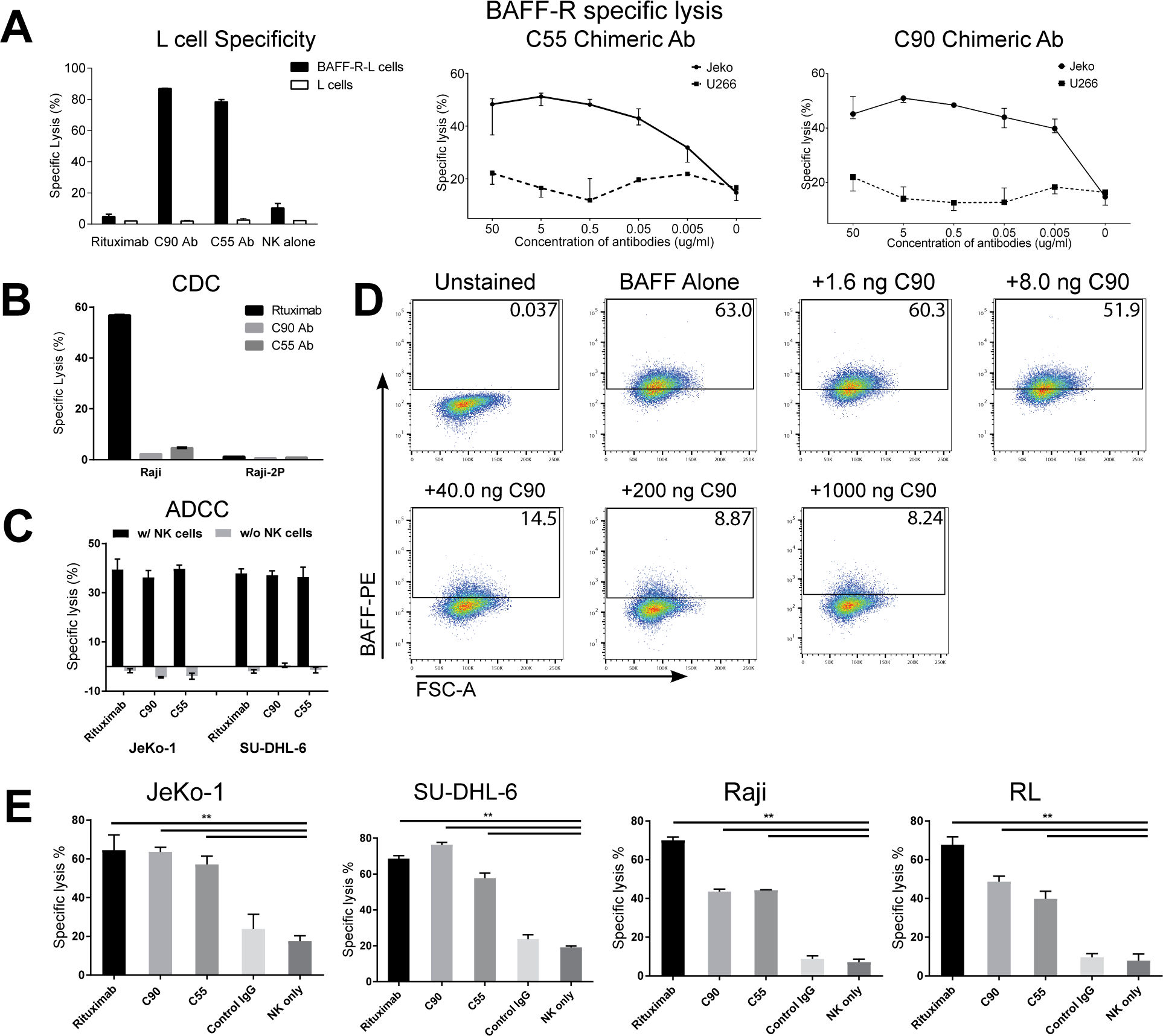
(A-D) Antibody BAFF-R specificity was determined by specific lysis and ligand blocking assays. Antibody-dependent cytotoxicity (ADCC) was measured by chromium-51 release assay and specific lysis of target cells was calculated as described in methods. Antibodies (C55, C90, or rituximab) and effectors (NK cells or complement containing serum) were incubated with chromium-51 labeled target cells. (A) Specific and dose response lysis are show for various target cells. BAFF-R expressing cells included BAFF-R L cells and JeKo-1, and BAFF-R negative controls include parental L cells and U266 human multiple myeloma cell line. NK cells were included in an effector to target ratio (E:T) of 20:1. (B) CDC specific lysis is show for CDC sensitive Raji and CDC-resistant Raji-2P. Active complement-containing human serum was incubated with target cells at 1:3 dilution. (C) ADCC specific lysis is shown for JeKo-1 and SU-DHL-6. Target cells and antibodies were incubated with or without effector NK cells (E:T = 20:1). (D) FACS plot gates show percentage of BAFF/BAFF-R binding signal in the presence of C90 mAb. BAFF-R-expressing D2C L cell clones were incubated with C90 (0–1000 ng/106 cells) at 4°C for 45 min followed by incubation with recombinant BAFF ligand (0.5 μg/106 cells) at 4 °C for 90 min. Flow cytometry was performed and gated for anti-BAFF-PE. (E) ADCC effects were measured on a panel of NHLs. Chromium-51 labeled NHL lines were incubated with antibodies rituximab, C90, C55, or Control IgG and effector NK cells (E:T = 20:1). NK cells only (without antibodies) was an additional control. Calculated specific lysis is shown as the mean ± s.d. of triplicate samples. **P < 0.05 compared with NK cell only and Control IgG by two-tailed Student’s t-test.
