Figure 4. Chimeric antibodies targeting human BAFF-R elicited in vivo therapeutic effects against B-cell tumors.
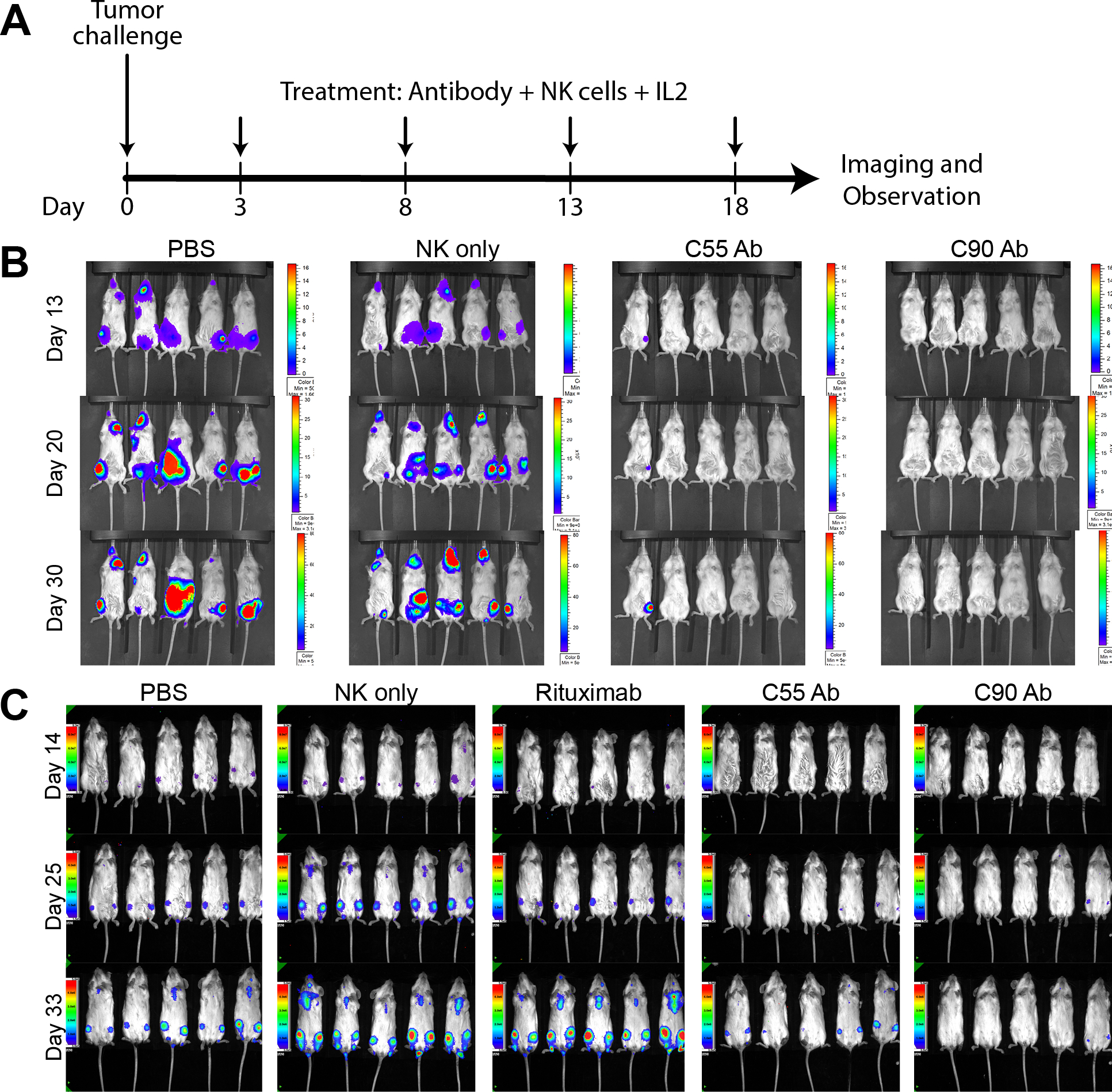
(A) Treatment schedule following Day 0 tumor challenge with minimum lethal dose of tumors. Antibody treatments were given by IV tail vein injections: 200 μg treatment antibody, 10 × 106 effector human NK-92-176V cells, and 5 × 104 IU IL-2. Bioluminescence imaging monitored mice challenged with luciferase-expressing tumors: (B) JeKo-1 (MCL) or (C) RS4;11 (ALL). Experimental groups received treatment of chimeric BAFF-R mAbs (C55 or C90, as indicated). Control group mice received PBS, NK cells alone, or rituximab on the same schedule. Data are representative of three independent experiments.
