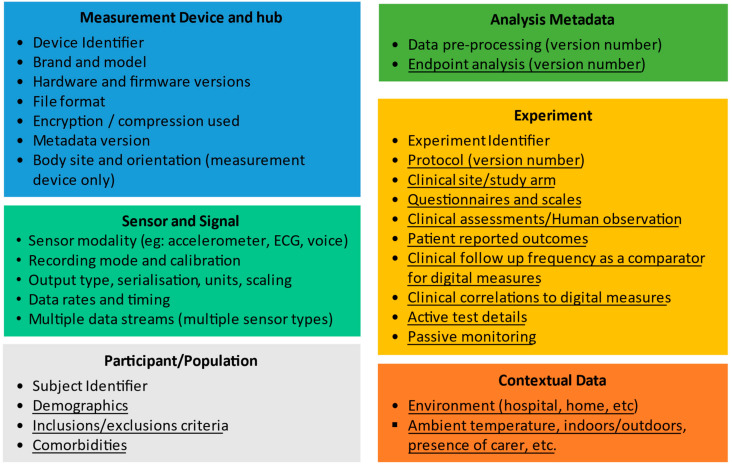
Figure 1.
A summary of the metadata elements needed to describe the collection of data from digital health technologies in a clinical trial setting. Underlined elements are application dependent and non-underlined items are application independent. The Patient ID and Experiment ID in the application-dependent metadata link to the application-dependent metadata. The “device” elements are required for the measurement device (e.g., a wearable) but also hub (might be a smartphone + app) that works with the wearable.