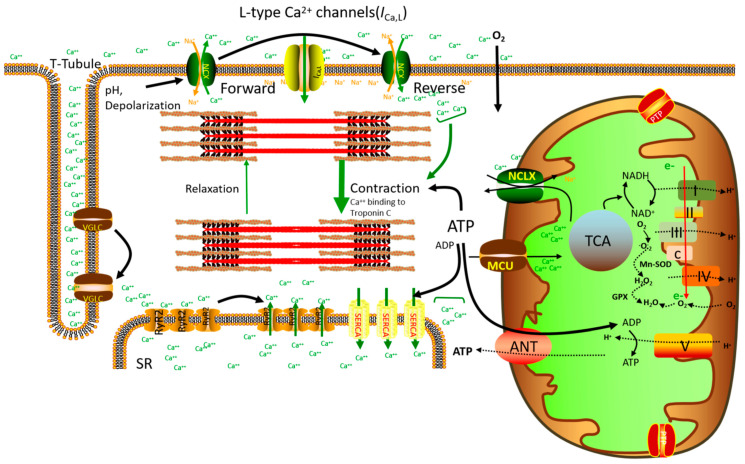
Figure 1.
Under physiological conditions, Ca2+ regulates excitation–contraction coupling, mitochondrial energetics, and ROS production in cardiomyocytes. In cardiomyocytes, action potential causes Ca2+ to enter cells from L-type Ca2+ channels (Ica, L), and the influx of Ca2+ activates Ryanodine Type 2 (RyR2) on the sarcoplasmic reticulum (SR), resulting in a large release of Ca2+ from the SR and subsequent binding to troponin C promoting myofilament cross-bridge formation, which causes cardiac contraction. During systole, Ca2+ enters SR via SR Ca2+-ATPase (SERCA) and exits the extracellular space via the Na+/Ca2+ exchanger (NCX). Mitochondria take up Ca2+ through MCU, and Ca2+ activate two key enzymes, isocratic dehydrogenase, and α-ketoglutarate dehydrogenase, of the TCA cycle and regenerate NADH+ from NAD. This causes electrons to move along the electron transfer chain (ETC) from complex I to complex IV. Complexes I, III, and IV pump protons (H+) into the intermembrane space, forming proton-motive forces bearing electrochemical potential and a proton gradient. Compound V converts ADP to ATP under proton drive. ATP is released into the cytoplasm through the adenine nucleoside transporters (ANT) on the inner membrane of mitochondria.