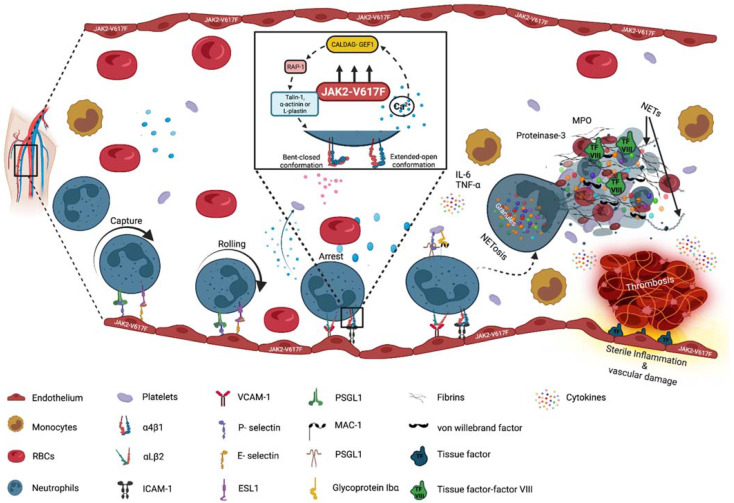
Figure 1.
Neutrophil-associated thromboinflammation in MPNs. The constitutive activity of mutated JAK2-V617F kinase increases intracellular Ca2+ and supports Ca2+ influx followed by CalDAG-GEF1 (calcium- and diacylglycerol-regulated GEFI) activation. This results in the activation of small guanosine triphosphate hydrolase enzymes (GTPases), such as RAS-related protein 1 (RAP1), which further stimulate integrin-binding proteins to facilitate integrin conformational changes in neutrophils. The activation of integrins assists neutrophils in inducing thromboinflammation, which is a multistep process where activated neutrophils, inflammatory cytokines, the aggregation of platelets, and induction of plasmatic coagulation synergize. Initially, under flow conditions, neutrophils interact with endothelium-expressed P- and E-selectin with their respective PSGL-1 and ESL1-ligands, which allow neutrophils to slowly roll along the blood vessel. While rolling, neutrophils are arrested upon the binding of β1 and β2 integrins (VLA-4, LFA-1) to the endothelium-expressed VCAM-1 and ICAM-1. Inflammatory cytokines, such as IL-6 and IL-17, foster this process by up-regulating VCAM-1 and ICAM-1 expression. The release of chemokines leads to chromatin decondensation in neutrophils, which then expel granular proteins, including neutrophil elastase (NE), myeloperoxidase (MPO), and DNA material into the extracellular space to form NETs. The formation of NETs further induces thrombosis by activating plasmatic coagulation and by inducing the aggregation of platelets and erythrocytes. (This figure was created with BioRender.com, assessed on 15 February 2022).