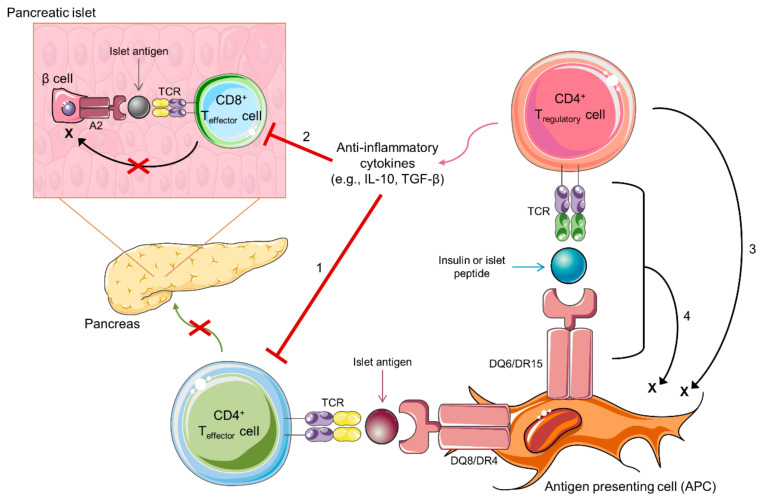
Figure 2.
Potential mechanisms for protection from type 1 diabetes development by antigen-specific regulatory T cells. Depicted is a CD4+ regulatory T cell (Treg) recognizing an insulin or islet peptide in the context of diabetes-protective HLA-DQ6 or DR15. This interaction leads to the production of anti-inflammatory cytokines (e.g., IL-10 and TGF-β), and the activation of these antigen-specific Tregs can protect from beta cell destruction by four potential mechanisms. (1) CD4+ effector T cells recognize islet antigens presented by diabetes-risk conferring HLA-DQ8 or DR4 on antigen-presetting cells (APCs) and then migrate to the pancreas where they induce pancreatic beta cell destruction. Antigen-specific Tregs can suppress CD4+ effector T cells and prevent the trafficking of these cells to the pancreas via the release of anti-inflammatory cytokines. (2) CD8+ T effector cells in the pancreatic islets recognize islet antigens presented by HLA-A2 on pancreatic β cells, leading to the destruction of the β cells. Tregs can suppress CD8+ effector T cells and prevent β cell destruction via the release of anti-inflammatory cytokines. (3) Tregs can target APCs via granzyme-mediated killing in an antigen-independent manner or (4) potentially in an antigen-dependent manner when the cognate self-peptide is presented to the Treg T cell receptor (TCR).