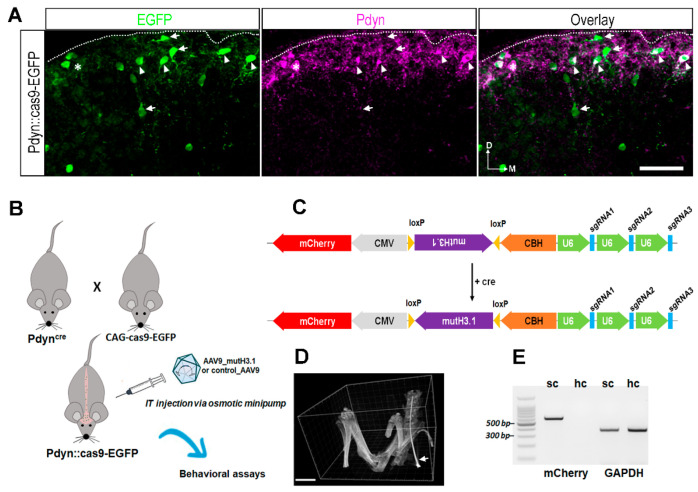
Figure 2.
Cell-type specific targeted delivery of the necessary components for genome editing of histone H3.1 via CRISPR/cas9 strategy. (A) Immunostaining with antibodies against EGRP (green), Pdyn (magenta) in a projected image of seven optical sections with a 40× lens from a transverse spinal cord section of a Pdyn::cas9-EGFP mouse. The overlay shows a merged image. The numerous EGRP-immunoreactive neurons are predominantly visible in the superficial layers of the lumbar spinal cord. The majority of these are Pdyn+ (arrowheads), while some of them lack Pdyn (arrow). Few Pdyn-expressing neurons lack the EGFP signal (asterisk). D, dorsal; M, medial; scale bar, 50 μm. (B) Schematic drawing representing the CRISPR/cas9 strategy to establish the mutant histone H3.1 (mutH3.1) in Pdyn neurons. The abbreviation mutH3.1 refers to serine-to-alanine exchange (S10A) at position serine 10 of the wild-type histone H3.1. IT, intrathecal; AAV9_mutH3.1, the mutant histone H3.1-containing recombinant adenoassociated virus serotype 9. (C) Schematic representation of the final insert synthesized and cloned into a recombinant AAV9. This cassette encoded mutH3.1 flanked by loxP sites (purple), three single-guide RNAs (sgRNAs; blue) driven by the human polymerase III U6 promoters (green), and a mCherry fluorescent protein (red). In this approach, S10A point mutation would be introduced into only cre-expressing neurons (i.e., into Pdyn-expressing neurons in Pdyn::cas9-EGFP hybrids). CMV, human cytomegalovirus (CMV) immediate early enhancer and promoter; CBH, chicken beta-actin promoter with CMV enhancer. For sgRNA sequences targeting wild-type histone H3.1, see Supplementary Table S3. See also Supplementary Data S1 for further technical details. (D) 3D volume reconstruction of micro-CT images, used for validating the intrathecal position of the inserted cannula before the osmotic pump implantation. The intrathecal catheter (arrow) is shown within the subarachnoid space in a living deeply anesthetized Pdyn::cas9-EGFP mouse. The catheter was introduced at the level of L5-L6 vertebral laminae and pushed up to L1-L2. Scale bar, 5000 μm. (E) In contrast to the hippocampus (hc), mCherry-specific RT-PCR produced a single sharp band in the spinal cord (sc) sample of a wild-type mouse that had been transfected with the AAV9_mutH3.1. GAPDH was amplified in both samples. BenchTop 100 bp DNA ladder was used as a reference.