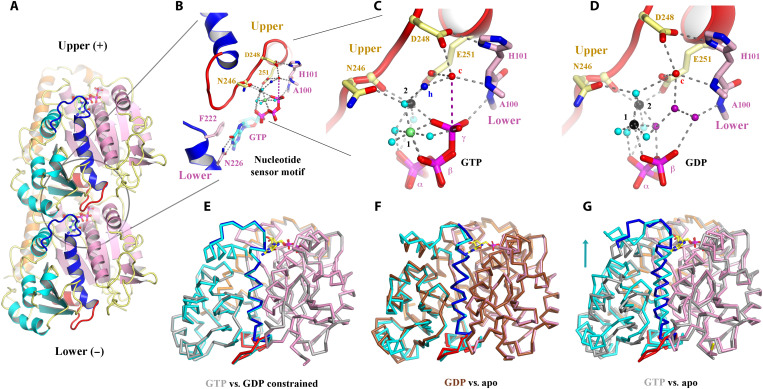
Fig. 2. Structural implications for GTP hydrolysis.
(A) The OdinTubulin protofilament in the crystal packing (PDB 7EVB). Two subunits of OdinTubulin are depicted. The α7 helix and preceding loop (dark blue) and α8 helix and preceding loop (red) comprise the nucleotide sensor motif, which connect the upper and lower GTP-binding sites (sticks). Secondary structure elements are colored by domain: N-terminal (pink), intermediate (cyan), and C-terminal (orange). The nucleotide sensor motif lies within the intermediate domain (see movie S4). (B) Enlargement of the GTP interactions. Only part of each nucleotide sensor motif is shown for clarity. Selected residues from the upper and lower subunits are labeled in yellow and pink, respectively. (C) Enlargement of the interactions around the GTP γ-phosphate. Black, lime green, and cyan spheres indicate Na+, Mg2+ (numbered in black), and water molecules, respectively. The proposed hydrolytic water is shown as a red sphere and labeled “c,” and water molecule suitably placed to receive the hydrogen ion from the hydrolytic water is labeled “h” in blue. The purple dashed line indicates the route for nucleophilic attack on the GTP γ-phosphate (see movie S5). (D) The same region from the GDP-bound structure (PDB 7EVE). Three water molecules (purple) replace the GTP γ-phosphate, and both cations are assigned as Na+ on the basis of bond distances and crystallization conditions (see movie S5). (E to G) Superimposition of protomer structures. (E) GTP-bound OdinTubulin (gray, PDB 7EVB) overlaid on the constrained GDP-bound structure (colored, PDB 7EVE). (F) The unconstrained GDP-bound OdinTubulin (brown, PDB 7F1B) overlaid on the apo structure (colored, PDB 7EVG). (G) GTP-bound OdinTubulin (gray, PDB 7EVB) overlaid on the apo structure (colored, PDB 7EVG). The arrow highlights the conformational change for the intermediate domain (see movie S7).