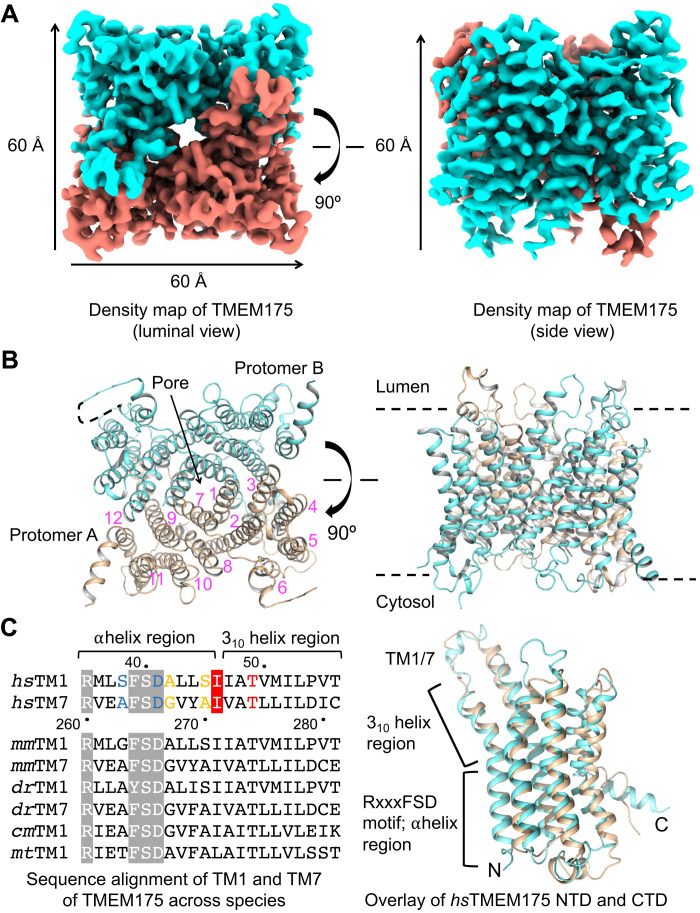
Fig. 4. The assembly mechanism of human TMEM175.
(A) 3D cryo-EM density of human TMEM175. Dimensions of the TMEM175 dimer are ~60 × 60 × 60 Å. (B) Ribbon diagrams of the human TMEM175 structure with the subunits colored in cyan and wheat, respectively. The 12 helices in one of the two subunits are labeled by numbers in magenta. (C) Sequence alignment of TM1 and TM7 across species and structural comparison of human TMEM175 NTD and CTD. In the aligned sequences, the conserved RxxxFSD motif is highlighted in gray, and the conserved gate residue is highlighted in red. Residues involved in K+ coordination are colored in blue, yellow, and red for hydrophilic layers 3, 2, and 1, respectively.