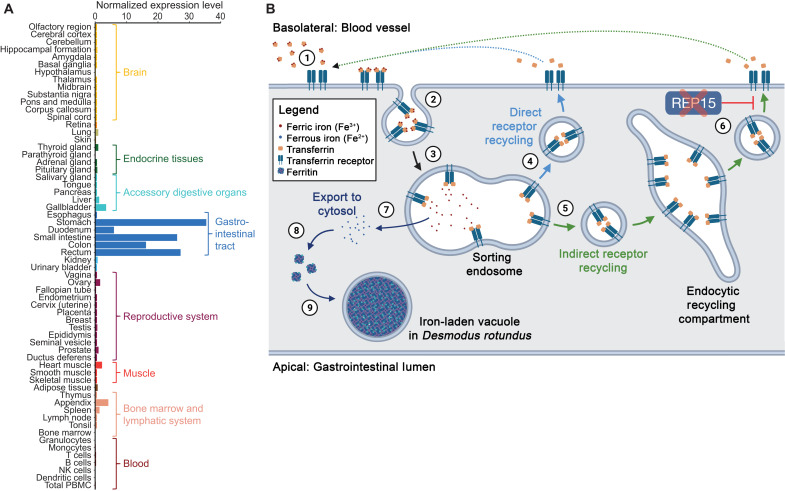
Fig. 4. Loss of REP15 in D. rotundus and enhanced iron excretion.
(A) REP15 mRNA expression is highest in gastrointestinal tract tissues. Data were taken from the Human Protein Atlas (www.proteinatlas.org/ENSG00000174236-REP15/tissue) (30) and show the consensus RNA expression values that integrate three gene expression datasets. (B) Illustration of transferrin receptor–mediated cellular iron uptake and function of REP15 in intestinal epithelial cells. Transferrin, an abundant ferric iron–binding plasma protein, binds to transferrin receptors that are present only in the basolateral membrane (1). Transferrin-transferrin receptor complexes are internalized via endocytosis (2). In sorting endosomes, ferric iron is released (3), and the unladen complexes are either directly targeted back to the cell membrane (4) or sent to the endocytic recycling compartment (5). REP15, encoded by the gene that is lost in D. rotundus, specifically localizes to the endocytic recycling compartment (6) and inhibits recycling of the unladen complex to the cell membrane (27), where transferrin and its receptor dissociate and the released transferrin can bind ferric iron again. Because the availability of transferrin receptors on the cell surface limits iron uptake (31), the presence of REP15 normally inhibits cellular iron uptake. In the sorting endosome, ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) and exported to the cytosol (7), where ferritin acts as the major high-capacity iron storage protein (8). Accumulations of ferritin and other “ferruginous” complexes enclosed in vacuoles (9) were observed in intestinal epithelial cells of D. rotundus (Fig. 3B). Loss of REP15 likely enhances iron accumulation in intestinal epithelial cells, and shedding of these cells boosts iron excretion in D. rotundus.