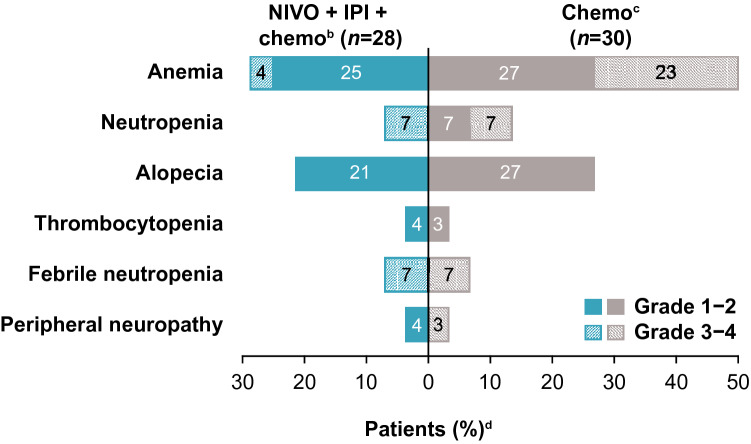
Fig. 3.
TRAEs typically associated with chemotherapya in the Asian subpopulation of CheckMate 9LA. aIncludes events reported between first dose and 30 days after last dose of study drug; bNivolumab plus ipilimumab combined with chemotherapy (2 cycles); cChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology); dData labels not shown for values < 1%. Chemo chemotherapy, IPI ipilimumab, n number of patients, NIVO nivolumab, TRAE treatment-related adverse event