Fig. 1. Study flow diagram.
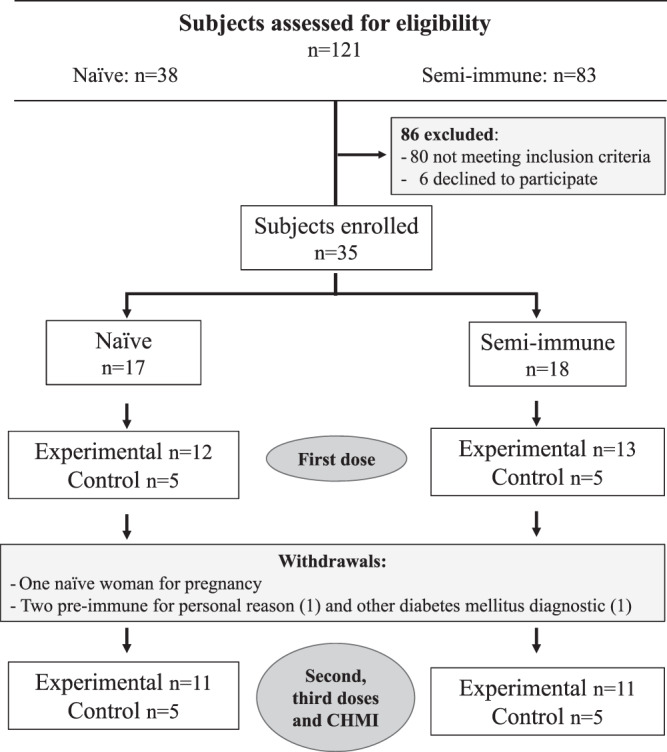
The study flow diagram describes the number of individuals in the screening, immunization, and CHMI steps. From 121 volunteers who initially accepted screening (38 naive, 83 semi-immune), 86 were excluded or declined participation and 35 were enrolled. Seventeen were naive and were allocated to Phase IIa study [12 Experimental (Exp) + 5 Control (Ctrl)], and 18 semi-immune to the Phase IIb study (13 Exp + 5 Ctrl). All 35 volunteers (age range 19–44 years) were immunized with PvCS LSP or placebo formulated in Montanide ISA-51. Two volunteers withdrew after the first immunization, and one more (semi-immune) was dropped out because of diabetes mellitus diagnosis.
