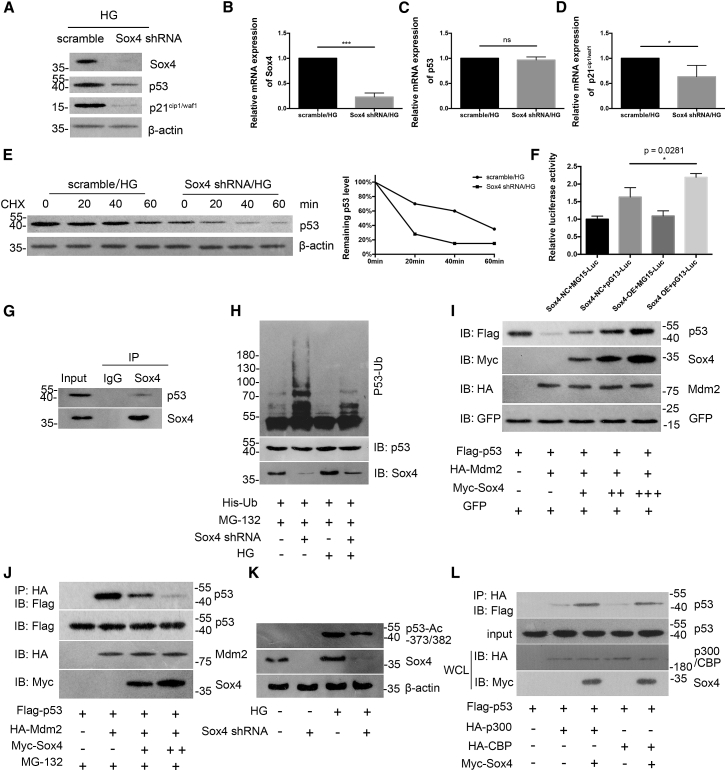
Figure 6.
Sox4 is critical for p53 stabilization and function
(A–D) Western blotting (A) and real-time PCR illustrated the expression of Sox4 (B), p53 (C), and p21cip1/waf1 (D) under HG conditions (n = 3). (E) Lysates from scramble shRNA or Sox4 shRNA-transfected podocytes treated with 20 mg/mL cycloheximide (CHX) at the indicated time points (0, 20, 40, and 60 min; left panel), and quantification of the relative p53 levels were quantified (right panel; n = 3). (F) Luciferase reporter assay revealed the interaction between Sox4 and p53 transcriptional activity in podocytes (n = 3). (G) Sox4 and p53 were endogenously expressed in podocytes, as shown by immunoprecipitation (IP) with an anti-Sox4 antibody (n = 3). (H) Podocytes were transfected with His-ubiquitin with or without Sox4 shRNA for 24 h and were incubated in the presence or absence of HG for another 48 h, followed by treatment with 20 μmmol/L MG-132 for 8 h. Cell lysates were immunoprecipitated and analyzed using immunoblotting with an anti-p53 antibody (n = 3). IB, immunoblotting. (I) Podocytes were transfected with FLAG-p53, GFP, HA-Mdm2, and Myc-Sox4 vectors (1, 2, and 4 μg) for 24 h, and the expression of p53 in podocyte lysates was analyzed using IB (n = 3). (J) Coprecipitation of p53 with Mdm2 was observed after Sox4 overexpression (2 and 4 μg) in podocytes in the presence of MG-132 for 8 h. Cell lysates were IP with an anti-HA antibody and were subjected to IB (n = 3). (K) Podocytes transfected with the control or Sox4 shRNA were treated with or without HG, and the levels of p53 acetylation at the Lys-373 and -382 residues were analyzed using IB with an anti-acetyl-p53 (Lys-373 or -382) antibody (n = 3). (L) Podocytes were transfected with FLAG-p53, Myc-Sox4, HA-CBP, and HA-p300 plasmids for 24 h. Cell lysates were IP with an anti-HA antibody and IB with an anti-FLAG antibody, and the whole-cell lysates (WCLs) were analyzed using IB (n = 3). Error bars represent ±SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.