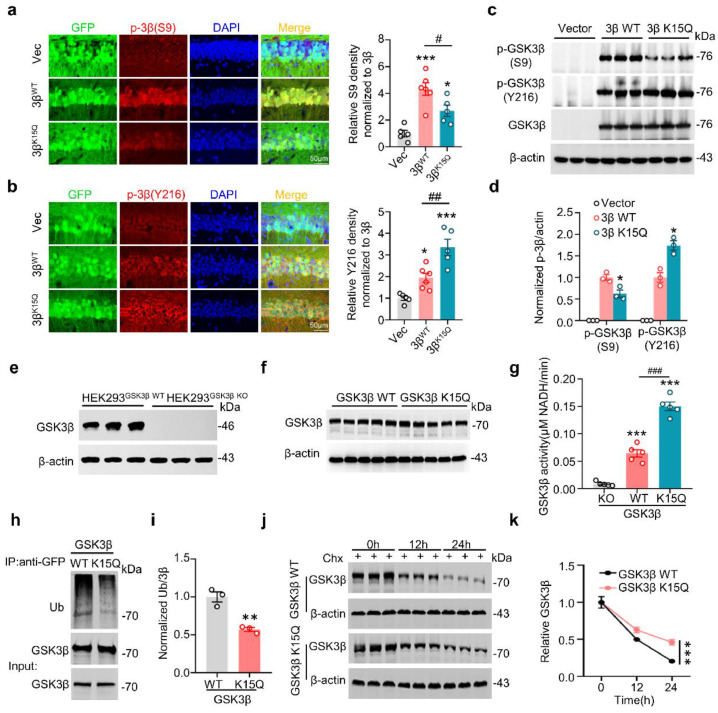
Figure 3.
GSK-3β K15-acetylation increases its kinase activity with reduced ubiquitination and proteolysis. (a,b) K15-acetylation increases GSK-3β activity in mice. AAV-GSK-3βWT or AAV-GSK-3βK15Q was stereotaxically infused into the hippocampal CA1 of 2-month-old C57 mice for 1 month, and then levels of pS9-GSK-3β (inactive) and pY216-GSK-3β (active) in CA1 were measured by immunofluorescence and normalized to total GSK-3β. (n = 5-6 for each group, one-way ANOVA, *p < 0.05, ***p < 0.001 vs Vec, #p < 0.05, ##p < 0.01 vs 3βWT, bar = 50 μm). (c,d) Empty-Vector, GFP-GSK-3β WT and GFP-GSK-3β K15Q plasmids were transfected into N2a cells, GSK-3β K15-mimic acetylation increased its kinase activity in N2a cells measured by Western blotting shown by decreased pS9-GSK-3β and increased pY216-GSK-3β (The band shows exogenously expressed GFP fusion protein). (n = 3 for each group, one-way ANOVA, *p < 0.05 vs GSK-3β WT). (e–g) GSK-3β K15-mimic acetylation increased its kinase activity measured by activity assay. GSK-3β was knocked out (KO) by using CRISPR/Cas9 assay and the expression of endogenous GSK-3β was verified by Western blotting (e), and re-expression of GFP-GSK-3β WT or GFP-GSK-3β K15Q plasmid in the KO cell model for 12 h and the exogenously expressed GFP fusion GSK-3β was verified by Western blotting (f) followed by GSK-3β activity assay (g). (n = 5 for each group, one-way ANOVA, ***p < 0.001 vs GSK-3β KO, ###p < 0.001 vs GSK-3β WT). (h,i) GSK-3β K15-mimic acetylation inhibited its ubiquitination. HEK293 cells were co-transfected with HA-ubiquitin and GFP-GSK-3β WT or GFP-GSK-3β K15Q plasmid for 24 h, and then GSK-3β was immunoprecipitated by anti-GFP and blotted by anti-Ub and anti-GSK-3β. (The band shows exogenously expressed GFP fusion protein). (n = 3 for each group, unpaired Student's t-test, **p < 0.01 vs GSK-3β WT). (j,k) GSK-3β K15-mimic acetylation inhibited its degradation. HEK293 cells were transfected with GFP-GSK-3β WT or GFP-GSK-3β K15Q plasmid for 24 h and then treated with cycloheximide (Chx,100 μg/ml) for 12 h or 24 h, and then measured the protein level of GSK-3β by western blotting. (The band shows exogenously expressed GFP fusion protein). (n = 3 for each group, two-way ANOVA, ***p < 0.001 vs GSK-3β WT, ###p < 0.001 vs GSK-3β K15R). Data were presented as mean ± SEM.