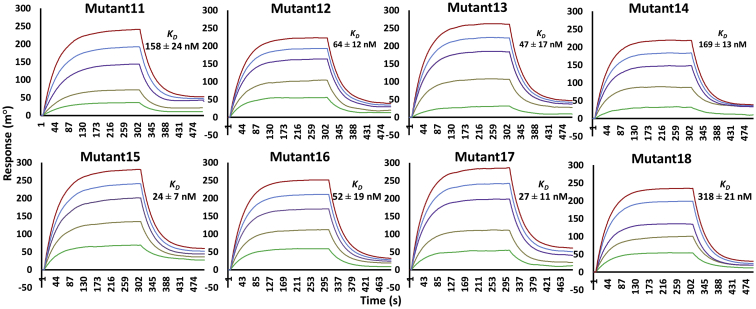
Figure 3.
Kinetics of scFv double and triple mutant proteins’ binding to the Fu-bc subunit protein. The Fu-bc subunit protein was coupled to the SPR biosensor chip, and scFv double (Mutant11–14) and triple (Mutant14–18) mutants were applied at graded concentrations (25–500 nM) over the coupled surface (from t = 0 to t = 300 s), followed by buffer washout (dissociation) for another 180 s and measurement of net binding (in RU). The association (ka) and dissociation rate constants (kd) were derived by fitting the recorded sensograms to (1:1) Langumir-binding rate equations, and the affinity constant KD was derived by dividing the dissociation constant (kd) with the association constant (ka). The overlay of curves for different concentrations of scFv protein was fitted into a numerical model by global analysis using Autolab kinetic evaluation software 5.1. The color of each fitted curve shown is a representative response of different concentrations of analytes (i.e., green, 25 nM; golden, 50 nM; violet, 100 nM; blue, 200 nM; and red, 400 nM). Fu-bc, fusion and bc loop; scFv, single chain variable fragment antibody; SPR, surface plasmon resonance.