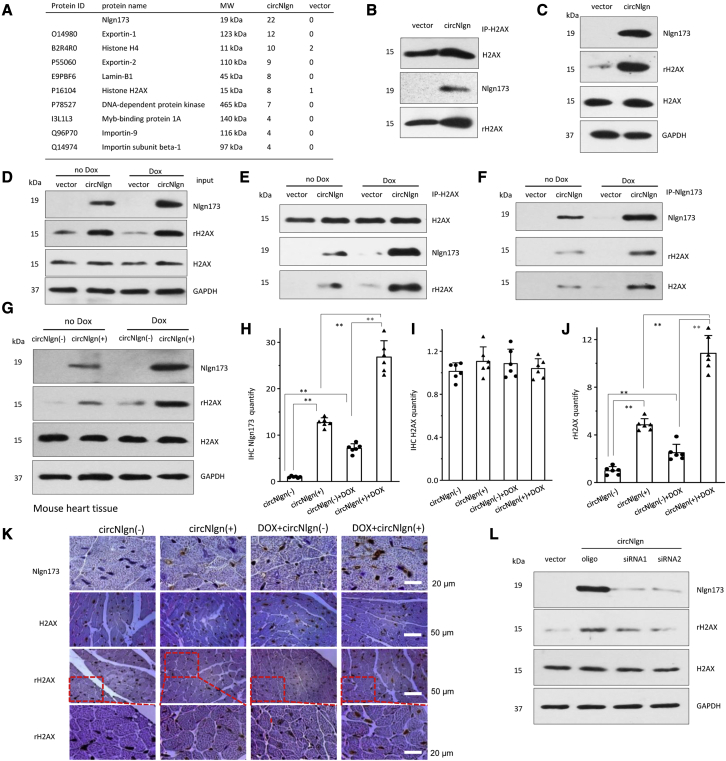
Figure 3.
Nlgn173 binds and activates H2AX, producing γH2AX
(A) Mass spectrophotometry showed proteins precipitated by antibody against Nlgn173. (B) AC16 cells transfected with circNlgn or the vector were lysed and subjected to immunoprecipitation with anti-Nlgn antibody followed by western blotting. Precipitation of Nlgn173 pulled down H2AX, and vice versa. (C) The interaction of Nlgn173 and H2AX promoted phosphorylation of H2AX (γH2AX). (D) AC16 cells transfected with circNlgn expressed higher levels of Nlgn173 and γH2AX, which were further enhanced by doxorubicin treatment. H2AX expression was not affected. (E) Precipitation of H2AX pulled down Nlgn173. H2AX phosphorylation (γH2AX) was higher in the sample where Nlgn173 interacted with H2AX. (F) Precipitation of Nlgn173 pulled down H2AX. The interaction of Nlgn173 and H2AX promoted phosphorylation of H2AX (γH2AX). (G) Western blot showed that circNlgn (+) mouse tissues expressed high levels of Nlgn173 and γH2AX, which were further enhanced by doxorubicin treatment. (H) Quantitation of IHC staining of heart tissues showed increased expression of Nlgn173 in the circNlgn transgenic heart, which could be further promoted by doxorubicin treatment. ∗∗p < 0.01 (n = 6). (I) Quantitation of IHC staining of heart tissues showed that H2AX expression was not affected by circNlgn expression nor by doxorubicin treatment. (J) Quantitation of IHC staining of heart tissues showed increased expression of γH2AX in the circNlgn transgenic heart, which could be further promoted by doxorubicin treatment. ∗∗p < 0.01 (n = 6). (K) Representative IHC photographs of heart tissues. circNlgn (+) mouse hearts displayed increased Nlg173 and γH2AX (Ser139) expression levels, which were further enhanced by doxorubicin treatment. H2AX expression was not affected. (L) Western blot showed that silencing circNlgn suppressed Nlgn173 and γH2AX expression.