Abstract
Anti-SARS-CoV2 mRNA vaccines showed a blunted antibody (Ab) response in people with MS (pwMS) on high efficacy therapies, suggesting the need for a booster dose. We evaluated the kinetics of the production of anti-receptor binding domain (RBD) Immunoglobulins G (IgG) after the vaccination cycle and the booster in pwMS receiving ocrelizumab, fingolimod and cladribine. A significant increase of anti-RBD IgG seroconversion was observed after booster respect to the vaccination cycle. Results obtained from this study will be useful for the management of pwMS in relation to their disease modifying therapy (DMT) and for any future vaccination campaign.
Keywords: Multiple sclerosis, Anti-SARS-Cov2 vaccine, Booster, Third dose
1. Introduction
Vaccination is indispensable to protect vulnerable people with MS (pwMS) from Coronavirus disease 2019 (COVID-19). Unfortunately, high-efficacy disease modifying therapies (DMTs) can interfere with immune response to anti-SARS-CoV-2 vaccination in pwMS through different mechanism of action: ocrelizumab depletes B cells by targeting CD20 and thereby may interfere in the process of antibody (Ab) production; fingolimod prevents lymphocyte egression from secondary lymphoid tissues by modulating SP1; the purine analogue cladribine selectively suppress T and B cells inducing severe lymphopenia (Achiron et al., 2021b). A blunted anti-Spike (S) Ab response elicited by anti-SARS-CoV-2 mRNA vaccines has been observed in pwMS treated with ocrelizumab and fingolimod, while cladribine showed an efficient Ab response (Achiron et al., 2021b, 2021a; Bigaut et al., 2021; Drulovic et al., 2021; Sormani et al., 2021). Despite the low Ab response following vaccination, discontinuation of highly effective DMTs has not been recommended due to the high risk of relapse (Giovannoni et al., 2021) and a booster dose was recommended. To date, data on booster have shown an adequate safety profile with a comparable adverse event incidence between the first vaccination cycle and booster (Dreyer-Alster et al., 2022). IgG levels increase after booster in untreated and treated patients (Dreyer-Alster et al., 2022), but data on B-depleting therapies and S1P modulators are not currently available. Here, we evaluated the effects of the booster in pwMS under high-efficacy treatments by measuring anti-receptor-binding domain (RBD) IgG titers, that can be considered as neutralizing Abs (nAb).
2. Methods
2.1. Patients
Subjects were recruited according to the following inclusion/exclusion criteria. Inclusion criteria: subjects diagnosed with MS according to the most recently revised Mc Donald's criteria and eligible for anti-SARS-CoV-2 vaccination. Exclusion criteria: any medical condition that did not allow the signing of informed consent. This study obtained ethics approval from the ethics committee of AOU San Luigi Gonzaga, Orbassano (TO), Italy; Ref. number #117-2021.
2.2. Serum collection
Sera were collected immediately before the first dose of Comirnaty vaccine (Pfizer/BioNTech Inc, BioNTech Manufacturing GmbH) (T0), 4 weeks (±7 days) after the first two doses of anti-COVID-19 vaccination (T1), before booster shot (6 months after vaccination ±15 days) (T6) and 4 weeks (±7 days) after booster (B) shot of Comirnaty or Spikevax vaccine (Moderna, Moderna Biotech Spain S.L.).
2.3. Anti-RBD IgG quantification
Anti-SARS-CoV-2 nAbs were measured with the SARS-CoV-2 RBD IgG ELISA (EIA-6150, RGD Diagnostic; lot number 142K061). Plates were read using iMarkTM Microplate reader (Bio-Rad). Results were expressed in IU/ml (log10), and the cut-off threshold corresponded to 1.4 IU/ml (log10), according to manufacturer indications.
2.4. Statistical analysis
Anti-RBD IgG levels were compared between each DMT group using analysis of variance (ANOVA) test. Adjusted P-value cutoffs of p≤0.05 indicated statistical significance. Data analyses were performed using Graphpad Prism version 8.2.1 (2019-08-18). A linear regression model, using R version 3.6.0 (2019-04-26), was used to compare IgG titers after booster considering age, sex, type of MS, EDSS, DMT, time between DMT and booster, type of booster (Comirnaty/Spikevax), lymphopenia grade (obtained from last total lymphocytes count within 3 months before booster) and pre-booster Ab levels.
3. Results
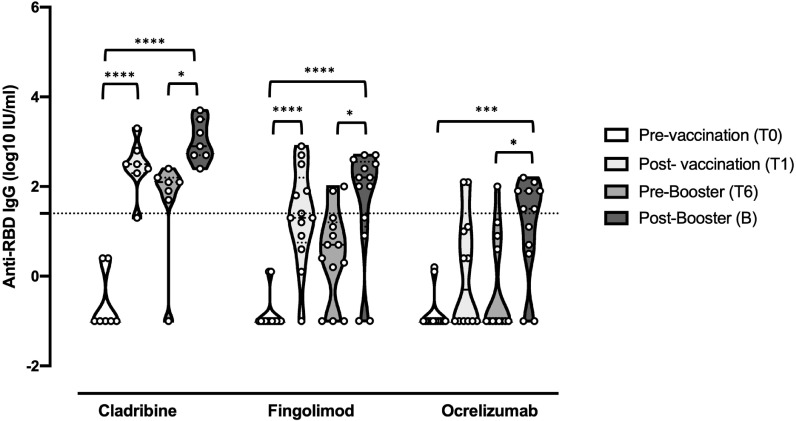
A total of 31 subject were recruited (12 on ocrelizumab, 12 on fingolimod and 7 on cladribine) and prospectively followed-up for 8 months. The demographic and clinical data of pwMS participating in this study are shown in Table 1 . COVID-19 disease was not reported from any of the subjects before vaccination, whereas 5 pwMS (1 on cladribine and 4 on ocrelizumab) had SARS-CoV-2 infection after the booster (median (IQR): 102 (57; 105) days) with mild symptoms and no hospitalization. The evaluation of anti-RBD Abs in the sera indicated that 85,7% of PwMS under cladribine, 41,6% under fingolimod and 16,6% under ocrelizumab treatment seroconverted after the first vaccination cycle. The booster increased the percentage of pwMS that seroconverted to 100%, 75% and 58,3%, respectively, showing an Ab response that, although attenuated, was above the cut-off value (Fig. 1 ). Furthermore, the levels of anti-RBD IgG after booster were higher respect to T6 where the nAb levels decreased (Cladribine T6 vs B, p= 0.014; Fingolimod T6 vs B, p= 0.025; Ocrelizumab T6 vs B, p= 0.010). By exploring the association of variables to post-booster nAb titers, we observed association with pre-booster nAb titers (coefficient = +0.52; p = 0.013) and the Spikevax booster (coefficient = 0.83; p = 0.09).
Table 1.
Clinical characteristics. Results are expressed as Median and Inter-quartile range (IQR).
| Cladribine | Fingolimod | Ocrelizumab | |
|---|---|---|---|
| Patients | 7 | 12 | 12 |
| Age | 49 (43.5; 53.5) | 50.5 (46.3; 56) | 56.50 (52.5; 60.3) |
| Female/male | 5/2 | 10/2 | 10/2 |
| RRMS/SPMS | 6/1 | 8/4 | 9/3 |
| EDSS | 3 (2; 4.8) | 2.25 (2; 6.5) | 5 (1; 6.1) |
| MS disease duration (years) | 9 (5; 14) | 18.5 (9.3; 24) | 10 (8.3; 22) |
| COVID-19 infections before vaccination | no | no | no |
| COVID-19 infections after vaccination |
no | no | no |
| COVID-19 infections after booster dose | 1/7 | no | 4/12 |
| Relapses between vaccination and booster dose | no | no | no |
| Relapses after booster dose | no | no | no |
| Time between vaccination and booster (days) | 161 (161; 180) | 165 (165; 180) | 207 (165; 216) |
| Time between therapy and booster dose (days) | 192 (161; 302.5) | N.A. | 138 (104.8; 220) |
| Booster type (Spikevax/Comirnaty) | 3/4 | 4/8 | 3/9 |
| Lymphopenia grade within 3 months before booster* | 1 (0,5; 2) | 2 (2; 2) | 0 (0; 1) |
Grade 3 = 200-499 lymphocytes/ul; grade 2 = 500-799 lymphocytes/ul; grade 1 = 800-999 lymphocytes/ul; grade 0 = >1000 lymphocytes/ul
Fig. 1.
Anti-RBD IgG titers before the first cycle of vaccination (T0), 4 weeks after (T1), 6 months after (T6), and 4 weeks after booster dose (B) in pwMS under high-efficacy DMTs. Dotted line corresponds to cut-off threshold of 1.4 IU/ml (log10). Asterisks correspond to p-values of ANOVA test (P<0,0001⁎⁎⁎⁎; P<0,0002⁎⁎⁎; P<0,002⁎⁎; P<0.03*).
4. Discussion
Here we evaluated anti-RBD IgG titers after booster in pwMS under high-efficacy treatments. The additional dose of anti-SARS-CoV-2 mRNA vaccine induced seroconversion of the majority of pwMS under fingolimod and ocrelizumab and of the totality of patients treated with cladribine, indicating that a booster dose could be fundamental in those pwMS that did not develop nAbs after the first vaccination cycle. A drop of nAbs have been shown within six months after the first vaccination cycle (Levin et al., 2021). Although a trend was visible, we did not observe a significant decay in anti-RBD IgG levels at six months, possibly due to a reduced response at one month after vaccination (Levin et al., 2021). Multivariate regression showed higher post-booster nAb titres in pwMS that had more pre-booster nAbs, and a likely greater efficacy of Spikevax than Comirnaty, as already observed after the first vaccination course in pwMS (Sormani et al., 2021). This could be due to the different dosage (Comirnaty booster contains 30µg of mRNA while Spikevax booster 50 µg of mRNA) or to heterologous vaccination. The 4 out of 5 SARS-CoV-2 infections observed after the booster occurred in pwMS treated with ocrelizumab that did not developed nAbs after the booster, accounting for the role of Ab response in preventing infections (Sette and Crotty, 2021). However, they efficiently eliminate the infection probably due to a preserved functionality of innate and T cells response (Giovannoni et al., 2021). Overall, the results reported in this study confirmed that cladribine treatment does not compromise the development of a specific humoral response, as previously observed (Achiron et al., 2021b; Dreyer-Alster et al., 2022); for ocrelizumab and fingolimod, a significant Ab response can be achieved with further vaccinations. Studies in larger cohorts, together with B and T cell-mediated responses profiling, will be needed to better clarify the effect of vaccination booster in pwMS in relation to their DMT and for any future vaccination campaign.
Author contribution statement
AM performed immunological experiments and wrote the manuscript; both MoM and RM recruited patients in the study and MoM contributed to sample preparation; MaM retrieved clinical data of recruited patients and performed clinical data analysis; MC conceived the study design and supervised clinical data collection and clinical data analysis; SR conceived the study design and supervised immunological experiments planning and analysis.
Declaration of Competing Interests
AM, MoM, RM and MaM have nothing to disclose; SR received travel grant from Sanofi-Genzyme; MC received personal compensations for advisory boards, public speaking, editorial commitments or travel grants from Biogen Idec, Merck Serono, Fondazione Serono, Novartis, Pomona, Sanofi-Genzyme and Teva.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank all the subjects enrolled in this study for their kind contribution.
References
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Dolev M., Menascu S., Magalashvili D., Flechter S., Givon U., Guber D., Sonis P., Zilkha-Falb R., Gurevich M. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. 17562864211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fabacher T., Lanotte L., Fleury M.-C., Collongues N., Seze J.de. Impact of disease-modifying treatments of multiple sclerosis on Anti–SARS-CoV-2 antibodies: an observational study. Neurol. - Neuroimmunol. Neuroinflammat. 2021;8 doi: 10.1212/NXI.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer-Alster S., Menascu S., Mandel M., Shirbint E., Magalashvili D., Dolev M., Flechter S., Givon U., Guber D., Stern Y., Miron S., Polliack M., Falb R., Sonis P., Gurevich M., Achiron A. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drulovic J., Ivanovic J., Martinovic V., Tamas O., Veselinovic N., Cujic D., Gnjatovic M., Mesaros S., Pekmezovic T. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Multiple Sclerosis Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C.H., Lechner-Scott J., Levy M., Yeh E.A., Baker D. COVID-19 vaccines and multiple sclerosis disease-modifying therapies. Mult. Scler. Relat. Disord. 2021;53 doi: 10.1016/j.msard.2021.103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 Months. N. Engl. J. Med. NEJMoa. 2021 doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Carmisciano L., Sormani M.P., Inglese M., Laroni A., Lapucci C., Uccelli A., Inglese M., Lapucci C., Uccelli A., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Battaglia M.A., Salvetti M., Franciotta D., Maglione A., Di Sapio A., Signori A., Laron A., Iovino A., Repice A.M., Mannironi A., Uccelli A., Serrati C., Nicoletti C.G., Lapucci C., Mancinelli C.R., Cordioli C., Bezzini D., Carmagnini D., Brogi D., Qranciotta D., Landi D., Orazio E.N., Cocco E., Signoriello E., Nako E., Assandri E., Marinelli F., Baldi F., Ansaldi F., Bovis F., Caleri F., Siciliano G., Cola G., Perego G., Lus G., Brichetto G., Icardi G., bellucci G., Rin G.D., Marfia G.A., Vazzoler G., Liberatore G., Trivelli G., Callari G., Gandoglia I., Maietta I., Schiavetti I., Frau J., Sticchi L., Pasquali L., Lorefice L., Carmisciano L., Ruggiero L., Manzino M., Bragadin M.M., Buscarinu M.C., Gagliardi M., Stromillo M.L., Sormani M.P., Ferrò M.T., Rilla M.T., Clerico M., Battaglia M.A., Ponzano M., Fronza M., Sette M.D., Inglese M., Scialabba M., Bedognetti M., Ulivelli M., De Rossi N., De Stefano N., Gazzola P., Bigi R., Dubbioso R., Reniè R., Iodice R., Fabbri S., Rasia S., Rolla S., Platzgummer S., Cordera S., Tassinari T., Carlini V. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]