Figure 3. NaV1.1 haploinsufficiency alters VPL neuron excitability.
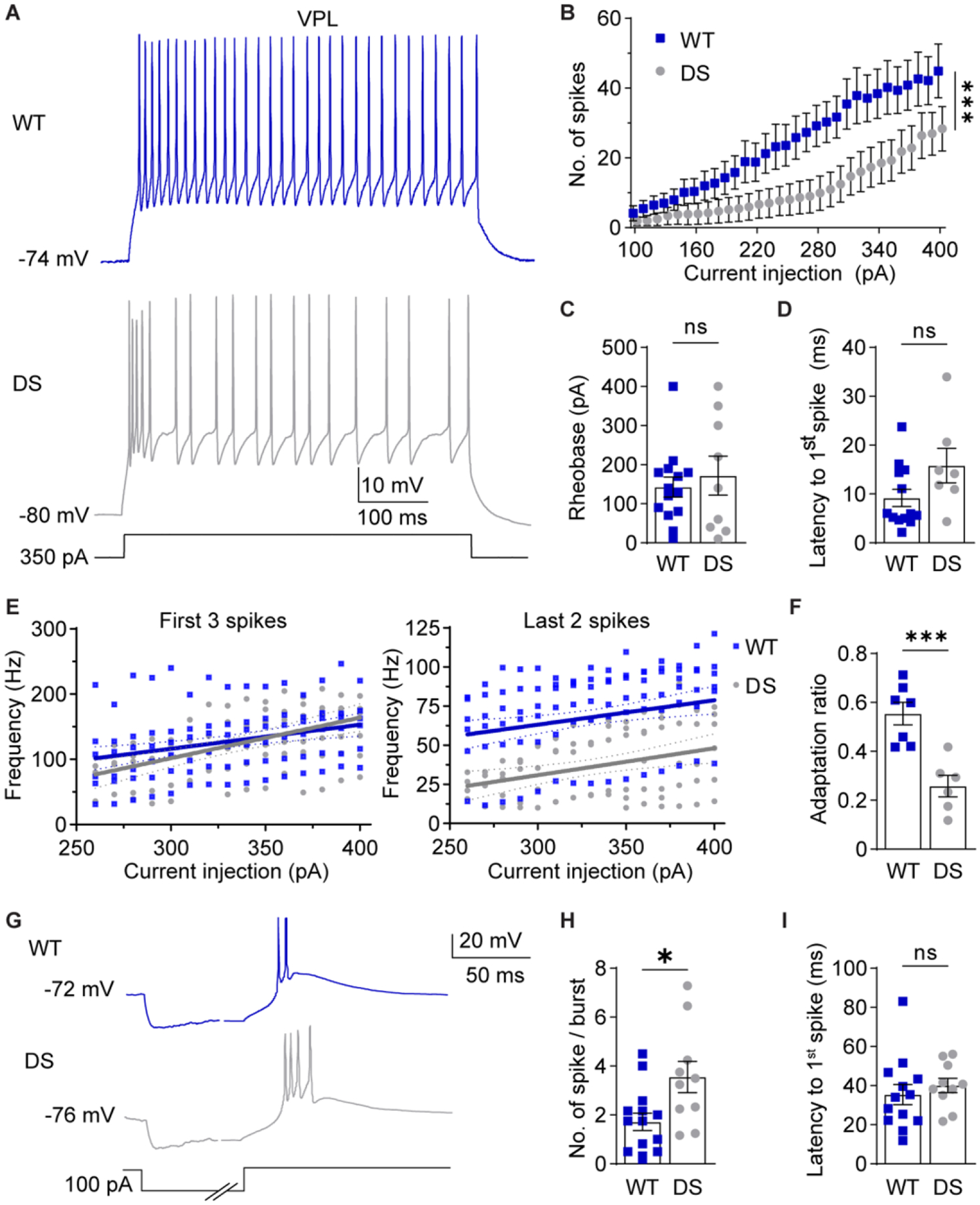
A. Representative traces show WT and DS VPL neuron spike firing in response to depolarizing current injections at RMP. B. The number of spikes fired by VPL neurons across current injections were analyzed by two-way repeated measures ANOVA (WT: n = 13 cells from 7 mice; DS: n = 8 cells from 6 mice; Genotype: F(1,19) = 3.605; p = 0.07, Interaction: F(30,570) = 2.200; ***p < 0.001) and posthoc Sidak’s tests at each current injection (p > 0.05 at each current amplitude). C. Rheobase was analyzed by unpaired t-test for WT (n = 14 cells from 7 mice) and DS (n = 9 cells from 6 mice) neurons (p = 0.571). D. Latency was analyzed by Mann-Whitney test (p = 0.183) due to failed normality (Shapiro-Wilk test, p = 0.026). E. The frequency of the first 3 spikes and last 2 spikes were plotted for each cell across current injections. Linear regression of WT and DS data yielded the plotted lines with 95% confidence interval (CI) bands, and fits were compared by sum of squares F tests. First 3 spikes: F (2,176) = 1.600, p = 0.205. Last 2 spikes: F (2,161) = 42.89, p < 0.001. F. Spike frequency adaptation ratios (last 2 spikes/first 3 spikes) were averaged across all current injections for each cell and compared by an unpaired t-test (***p < 0.001). G. Representative traces show rebound burst firing at RMP upon recovery from hyperpolarization. The time axis was broken to facilitate displaying the hyperpolarization and spike periods. H. Spikes per burst (*p = 0.01) and (I) burst latency (p = 0.49) were compared by unpaired t-tests (WT: n = 13 cells from 7 mice; DS: n = 10 cells from 6 mice). The symbols in all bar graphs
