Abstract
Background
Women living with HIV who misuse alcohol and live in economically disadvantaged settings in South Africa experience a multitude of contextual barriers as they navigate the HIV care continuum. The Women's Health CoOp (WHC), a brief, woman‐focused, behavioural, evidence‐based intervention, has been shown to be effective in reducing heavy drinking and improving HIV‐related outcomes among this key population. However, these women face other broader socioecological barriers to antiretroviral therapy (ART) adherence.
Methods
The WHC was implemented in a modified, stepped‐wedge implementation science trial in public health clinics and substance use treatment programmes in Cape Town, South Africa. A qualitative substudy was conducted to explore barriers to HIV treatment adherence among women enrolled in this trial. Eight focus group discussions were conducted with 69 participants 6 months after completion of the WHC workshops. Focus groups were audio‐recorded (with consent), transcribed verbatim and analysed using a thematic approach.
Results
The mean age of the participants was 33 years and the mean self‐reported number of drinks per day was 13. The main contextual factors influencing participants’ ART adherence were intrapersonal‐level factors (substance use, financial constraints, food insecurity; community‐level factors (anticipated and enacted stigma, community violence) and institutional‐level factors (patient–provider relationships, health facility barriers, environmental stigma).
Conclusion
Comprehensive interventions addressing the contextual barriers and unique challenges faced by women who misuse alcohol in low‐resource settings that intersect with HIV treatment nonadherence should be implemented in tandem with successful biobehavioural HIV interventions for long‐term effectiveness and sustainability.
Patient or Public Contribution
Our South African community collaborative board has been involved throughout this study; participants and clinic staff voices have been essential in our interpretation of these findings.
Keywords: alcohol and other drugs (AOD), antiretroviral therapy (ART), gender‐focused, HIV care continuum, implementation science, socioecological barriers
1. INTRODUCTION
In 2016, South Africa adopted the World Health Organization's Universal Test and Treat policy, making all people living with HIV eligible for antiretroviral therapy (ART) at diagnosis. 1 However, despite successful scale‐up of HIV testing and treatment, suboptimal retention in care and poor viral suppression through nonadherence to ART continues to be a major challenge. 1 Women aged 25–49, experience a disproportionate burden of HIV prevalence (33.3%) as compared with men (19.4%); however, only 69%–75% of women on ART are virally suppressed, which is short of the UNAIDS 95‐95‐95 targets by 2030. 2 , 3
Various intrapersonal‐level factors have been identified to help understand the causes of HIV treatment nonadherence and barriers that women living with HIV face as they try to remain in HIV care. One barrier of concern is substance use, which has been associated with reduced HIV adherence and HIV disease progression. 4 , 5 , 6 Estimates suggest that in 2012, approximately 2900 HIV‐related deaths and 11,400 years lived with a disability among women living with HIV in South Africa were attributable to substance use and its effect on nonadherence to ART. 7 Other contextual barriers to ART adherence among women living with HIV include gender‐based violence and a history of social, legal, and economic disempowerment and gender inequality that impact women's ability to engage in care. 8 , 9 , 10 , 11 Consequently, the intersecting syndemic of HIV, substance use and gender inequality necessitates multifaceted interventions to mitigate the risk of nonadherence and poor health outcomes among women on the individual level. 10 , 12 , 13
The Women's Health CoOp (WHC), a brief, woman‐focused, behavioural, evidence‐based intervention, grounded in empowerment and feminist theory is one such intervention. The WHC uses a skill‐building approach to reduce varying risk behaviours, including alcohol and other drug (AOD) use, sexual risk behaviour, gender‐based violence and biomedical knowledge of HIV and sexually transmitted infections (STIs). Based on an intervention developed in the US for African American women who use substances and adapted to various key populations and settings, the WHC has been found to be efficacious in reducing HIV risk for women who use AODs. 9 , 14 , 15 In a recent cluster‐randomized trial of the WHC with biobehavioural approaches, women in the WHC arm had greater reductions in heavy drinking and other risk‐related outcomes, and greater reductions in HIV viral load were observed for a subsample of WHC participants living with HIV. 14
However, addressing the nexus of substance use, gender inequality and HIV is only part of a larger socioecological framework. Other factors that converge with substance use, such as food insecurity and financial constraints, have been found to be strong predictors of suboptimal HIV treatment outcomes. 16 , 17 , 18 Additionally, stigma, access to healthcare and institutional and health‐system factors may undermine the sustainability of successful behaviour change evidence‐based interventions focused on ART adherence. 19 , 20 , 21 The present study explored how multiple factors of the socioecological framework impact HIV treatment and adherence among women living with HIV who use AODs.
2. METHODS
This qualitative study was part of a larger implementation science research study to evaluate the effectiveness of the WHC for women living with HIV and who reported AOD use in Cape Town, South Africa. 10 , 22 The WHC intervention included two interactive group workshops that combined risk‐reduction information about AODs, ART initiation, understanding the importance of ART adherence and STIs. Workshops also included material on behavioural skills training, such as practicing male and female condom use, and reducing sexual risk through negotiation and communication skills. The WHC was implemented in four public healthcare clinics with HIV/antenatal clinics and four substance use treatment clinics (hereafter known as Matrix programs), all located in economically underserved communities in Cape Town, from 2015 to 2018 using a modified stepped‐wedge design of four cycles. 10 , 22 Each implementation cycle lasted 6 months. Focus group discussions were conducted with study participants approximately 6 months after women had attended the WHC workshops to assess the barriers to ART adherence from a socioecological framework.
2.1. Recruitment and data collection
The 480 women who participated in the WHC implementation science trial met the following eligibility criteria: (1) being between the ages of 18 and 45 years; (2) self‐reporting the use of at least one drug, which could include alcohol, at least weekly during the previous 3 months; (3) reporting unprotected sex (sex without a condom) with a male partner in the past 6 months; (4) having a positive verifiable HIV test result; (5) reporting the intention to remain in the study area for at least the next 6 months; (6) providing contact information and (7) being willing to participate in AOD use screening. 10 An additional criterion for participation in the focus group discussions was the completion of both WHC intervention workshops, which 84% of trial participants met. Eligible participants from each of the eight study clinics in which they were enrolled were randomly within each cycle selected and contacted for the follow‐up focus group discussions. A total of 110 women were contacted from across the cycles, with 69 attending the focus group discussion. We conducted eight focus group discussions—one focus group discussion from each of the eight clinics consisting of between 5 and 11 participants in each group (median = 9). Each focus group lasted approximately 1 hour.
All focus group discussions were conducted in a private room at the research study site. Participants provided written and signed informed consent before each focus group. Focus group discussions were primarily conducted in English by trained research multilingual project staff, some of whom acted as real‐time interpreters when translations in isiXhosa and Afrikaans, two of the primary languages spoken in Cape Town, were needed. These staff were trained in understanding the focus group questions and the ability to translate the meaning of participants’ answers and comments as discussion happened. Focus group discussion guides were semistructured, consisting of open‐ended questions and prompts to explore multiple topics, including participants’ feedback on the WHC workshops and behavioural and contextual factors that influenced their ART adherence, such as barriers experienced as participants tried to navigate through HIV treatment.
2.2. Analysis
All focus group discussions were audio‐recorded (with consent) and transcribed by trained research staff. A second staff transcriber reviewed recordings and transcripts to ensure completeness. We used an applied thematic analysis approach to guide analysis, 23 we began by conducting a deep reading of the transcripts to familiarize ourselves with the data and wrote memos to identify recurring concepts. We developed an initial codebook using a priori codes based on the Focus Group Discussion guide and common concepts observed during transcript review. Interrater reliability was assessed using Cohen's kappa (κ). 24 One analyst applied codes to the transcripts and the other analyst coded the same transcript blinded to the first analyst's codes. Analysts then met several times to compare coding, refine code definitions and resolve disagreements. On reaching a high agreement (κ = 0.81), the remaining transcripts were double coded by the two analysts. Coded data were summarized in visual matrices to identify themes within and across focus group discussions. Dedoose software (v.8.0.42) was used for the management, coding and analysis of the data.
2.3. Ethics
This study protocol was approved by the South African Medical Association Research Ethics Committee (SAMAREC); City of Cape Town: City Health Research Committee, and the RTI International Committee for the Protection of Human Subjects.
3. RESULTS
The mean age of the participants in this substudy was 33 years (see Table 1). A majority of the women were Black African (94%) and 84% reported having a male partner and an average of two children. Although over two‐thirds of the participants had completed Grade 9 and above, only 5% had completed high school (Grade 12). Approximately 45% of the participants did not have running water in their homes. The mean self‐reported number of drinks per day was 13. The main contextual factors influencing participants’ ART suboptimal adherence found in the analysis are presented under three components of the socioecological framework: intrapersonal level, community level and institutional level (Figure 1).
Table 1.
Selected participant characteristics
| Total (N = 69) | |
|---|---|
| Mean age (years) | 33 (SD = 6.0) |
| Black African | 94% |
| Have a main partner | 84% |
| Number of children (mean) | 2 (SD = 1.3) |
| Education completed | |
| Grades 1–8 | 29% |
| Grades 9–11 | 66% |
| Grade 12 | 5% |
| Living condition | |
| No running water inside the house | 45% |
| House whose walls is made of metallic sheet | 45% |
| Gone to bed hungry at least once in the past year | 34% |
| Substance use | |
| Mean drinks per day in the last 30 days | 13 (SD = 7.0) |
Figure 1.
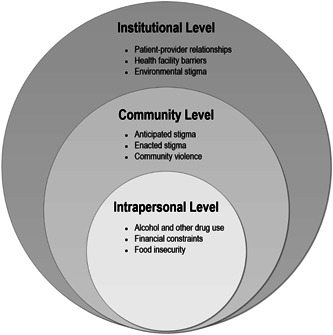
Primary reasons identified for nonadherence to antiretroviral therapy among women living with HIV
3.1. Intrapersonal level
3.1.1. AOD use
AOD‐use−related barriers were a recurring theme. For some participants, AOD use took precedence over engagement in care. Women reported their struggle with substance use and an inability to attend their clinic appointments to get their medication.
I was drinking too much, hence I decided to stop taking my treatment…. I did not to go back to the clinic because I was not taking the medication…. (Matrix program)
Some women intentionally did not adhere to ART while drinking alcohol because of interactive toxicity beliefs. Participants believed that because both ART and alcohol are drugs, mixing them would lead to adverse health outcomes.
I was told that the ARVs are [a] drug so I did not take medication when I am going to drink alcohol. I would not mix a drug with another drug because I don't know how they are going to affect my body. (Matrix program)
Toxicity beliefs often stemmed from clinic staff who emphasized alcohol abstinence but concurrently provided conflicting messages on the safety of taking ART while using AODs.
They are confusing us at the clinic because some are saying we must not take our medication if we will be drinking alcohol on that day, then some will say take them before drinking alcohol and wait at least 4 to 5 hours before you start drinking. (HIV/antenatal clinic)
These conflicting messages resulted in women not taking their ART over the weekends when their drinking levels were higher.
3.1.2. Financial constraints
Unemployment and lack of income were reported as a significant challenge to ART adherence. Some participants felt they were not financially stable enough to responsibly commit to taking daily medication and were dependant on their male partners or family for assistance.
I can't depend on someone else for…my treatment. At the end of the day, if I don't eat … I'm gonna get dizzy, or whatever. So that's like starting something that I won't be able to cope with, you understand? But if I had money… then I would say, yeah, I can buy my own groceries every month. No one can tell me I must thank my mother and my father and stay with my fiancé. (HIV/antenatal clinic)
Limited resources led to competing demands. As women who used substances, participants reported they often had to choose between buying substances and providing for other needs, such as food, when they secured some money.
I will just give you an answer. If … you have got those two things [money and HIV], and like you're a user like me. Definitely you know what about choices. Well yes, I'm going to go buy my substance, not food. (Matrix program)
Lack of resources for transportation presented a barrier to health service utilization. Participants expressed difficulty in securing money to pay for public transport to the clinic for their appointment.
It's far [the clinic]. Some of us we are not working. We do not have money for transport [transportation]. (Matrix program)
For some participants who qualified for government financial assistance programmes through disability grants—a lifeline for ART patients that enables them to meet healthcare‐related costs, including transportation, food and treatment access—this assistance was short‐lived as they would lose their grant once their health had rebounded.
And the treatment that we take makes us very hungry and we cannot take treatment on an empty stomach. They refuses to register us for the grants, they will just look at us and assume that we OK or doing good, and we struggling not working. They only give you a grant when you are really sick. (Matrix program)
3.1.3. Food insecurity
Though mentioned sometimes in relation to financial insecurity, food insecurity was another reason for treatment interruption. Participants reported worsening treatment side effects when they took ART without food, leading them to discontinue their medication.
It's about food. (Group ‘Mhm’). ‘Cause if you take that medication and then from that you get sick…it makes you hungry. I don't like to get sick and I don't like to drink that medication. (Matrix program)
Sometimes you don't have food at home…. How can we take care with an empty stomach? (HIV/antenatal clinic)
Participants reported frustration and helplessness because of a lack of food; consequently, for some, the belief in the inevitability of death because of one's HIV status was adopted as a coping mechanism.
I have defaulted quite a number of times. I sometime[s] think that I am just wasting my time because I am going to die anyway because I am taking the medication on an empty stomach. (Matrix program)
3.2. Community level
3.2.1. Anticipated stigma
Fear of community stigma because of one's HIV‐positive status dissuaded participants from seeking HIV care. Participants reported avoiding going to their clinic for HIV services because they would be recognized by a community member, inadvertently disclosing their HIV status.
Because … sometimes we know each other from the location, and it's obvious when I see [you] here I will conclude that you ‘Nice‐nice’ [living with HIV]. (HIV/antenatal clinic)
Participants shared their concerns about disclosing their HIV status to their partners and family members.
Others can't share with family that they are HIV positive because they are afraid of being discriminated. Others don't share their status with their partners so that makes it difficult for them to take the medication. (HIV/antenatal clinic)
3.2.2. Enacted stigma
Other participants reported personal experiences of the negative consequences of disclosing their HIV status to their family members, which impacted their health‐seeking behaviour.
When they found out that I was HIV positive at home…my own utensils…were always in jik [chlorine bleach] water. They did not even like my child because she was HIV positive and she [was given] to the social workers at the hospital. (HIV/antenatal clinic)
Stigmatizing language and demeaning attitudes held by clinic staff drove away participants from continuing with their healthcare.
They talk to you anyhow, they are sometimes rude to you…it's like when you get infected ne, it's like it was a choice but it's never been a choice. No one goes around looking for HIV. But that is how you are treated at the clinics. (HIV/antenatal clinic)
Participants also reported mistreatment from clinic staff because of missing appointments, which impinged on adherence to medication.
If you have missed your date, they treat you very bad and shout at you. So that makes one feel…scared to go back to the clinic… you end [up] defaulting. (Matrix program)
3.2.3. Community violence
For a few women, fear of violence in the community acted as a physical hindrance to attending the clinic appointment.
So like mine [adherence] has also [gone] down now because for months [I have been] out of my tablets…because…I am in a situation now. Understand? I can't go to the clinic there by me…because…if I'm going to walk on my own because [I might] witnessed a murder. So, I am now home without my tablets. (Matrix program)
Similarly, fear of being robbed or physically harmed for their ART medication forced some women to avoid going to the clinic.
And then when they know what kind of medication you are carrying from the clinic; the gangsters start robbing you by taking your ARV medication by force. (HIV/antenatal clinic)
3.3. Institutional level
3.3.1. Patient–provider relationship
Participants reported that clinic staff were dismissive and did not provide adequate information during HIV Counselling and Testing (HCT) concerning their health. They revealed that staff did not show concern nor empathy and they felt unsupported. At times, important HIV knowledge was only imparted after they had defaulted from their treatment. Participants reported feeling disempowered and unable to achieve their health goals.
They test you and tell you that your blood is gone to the lab, when you come back for your date, they just tell you that ‘you OK’. They always say your bloods are ‘OK’, without explaining about some other things. They won't even give a chance to ask more or for any other things, they just say ‘Sisi you OK’, only when you defaulted they send you to the counsellors, you get counselling…. (HIV/antenatal clinic)
Participants reported they were afraid to be honest about their ART adherence because of social desirability that resulted from their clinicians’ zero‐tolerance approach. Some participants reported sharing their missed medication with others to appear to have completed their dose during their follow‐up clinic appointment.
Sometimes you skip a day or 2 not drinking [taking] treatment. So I think you can give out that one you were supposed to take but you skipped… at the clinic when they count it's going to look like you are up to date with your treatment, you have been taking accordingly [laughs]. We all doing it [laughs]. (HIV/antenatal clinic)
Any mechanism for expressing patient satisfaction and redressing corrective action in the clinics, such as suggestion boxes and books, seemed to be defunct. Patients felt discouraged to give feedback as this was often ignored. At times, those who spoke up faced negative consequences, such as being seen last or experiencing rude behaviour.
They are always rushing…. We won't see any change and when you start complaining they will tell you [are] being rude to them, shift your folder for you to be help[ed] last or send you to another sister. The sisters are very rude. (HIV/antenatal clinic)
3.3.2. Health facility barriers
Participants reported they experienced difficulties in refilling their medication when they moved to a new community that was not served by their clinic or if they moved to a new town. The lack of integration of the health facilities' health records frustrated the participants, such as policies where a transfer letter was needed to initiate treatment in a new clinic.
Sometimes you move to another province. When you get there, they will ask for a transfer letter. They can't give you medication if you don't have the transfer letter. (Substance use treatment rehab clinic)
Some clinic administrative barriers hindered women's ability to engage in HIV care. Most clinics did not require appointments. However, women reported that when they went in early to be among the first patients, they could not complete their appointment because of administrative setbacks. One participant described her experience of waiting an entire day to be seen by a provider.
Sometimes I get to the clinic at 7 [in the morning] then I wait until 4 [in the afternoon] and every time I go and ask…they will tell me that my folder is missing, I must sit down and wait, and that's not motivating for me. I would go back home without my medication because of the missing file. (HIV/antenatal clinic)
3.3.3. Environmental stigma
Fear of unintended disclosure was reinforced by various structural characteristics in the clinic environment—such as conspicuously demarcated HIV‐related service departments and different coloured medical records for HIV‐related care—giving a clear indication of what services one was receiving.
[The] thing at the clinic [is] the sections there have different colours on the walls, like people knows if you go into the pink room, you are there for HIV treatment and the yellow room is for something else. (HIV/antenatal clinic)
For some participants, this clinic environment led them to seek care in clinics that were outside of their communities.
4. DISCUSSION
The overarching goal of the evidence‐based WHC is to empower women to take better care of their health by reducing alcohol use and adhering to ART. However, although the WHC has been shown to be effective, 22 there were, in a broader context, barriers to ART adherence and retention in care among women who had completed this intervention. Emerging themes of this substudy revealed these interwoven socioecological factors.
We found that at the individual level, AOD use was the most salient reason for ART interruption. Previous studies have found that individuals who used AODs were more likely to have low adherence 6 months following ART initiation 25 where the comorbidity of substance use disorders reduces self‐efficacy and self‐care, leading people living with HIV to disengage in healthcare. 26 Additionally, interactive toxicity concerns between substance use and ART medication have been shown to adversely impact ART adherence. 27 , 28 These toxicity concerns often lead to intentional nonadherence or treatment interruptions where people living with HIV stop taking their ART during certain periods when they are using AODs, compromising optimal ART adherence and increasing the likelihood of medication drug resistance. Healthcare providers often discourage substance use while on ART, stressing that substance use undermines the effect of ART. 29 However, research suggests that the pharmacological effectiveness of ART is not diminished by substance use. 30 Consequently, educating providers on the minimal impact of substance use on ART efficacy may decrease ART toxicity beliefs and increase ART adherence among those who use substances. Providing further resources for substance use counselling and treatment also may be more effective in increasing ART adherence than exclusively focusing on abstinence. 22 , 31 , 32
It also is well documented that the HIV disease burden among those of lower socioeconomic status is disproportionally high. 33 , 34 These findings indicate that lack of financial resources caused by poverty and unemployment negatively affect participants’ ability to engage in HIV care. First, although participants wanted to initiate and remain on treatment, ART became an added obligation where financial constraints already existed. Competing priorities, particularly in low‐resource settings where financial insecurity presents numerous challenges, may lead to nonadherence. 18 , 33 , 35 , 36 , 37 , 38 Second, although ART is free in public health clinics in South Africa, the financial costs of reaching the clinic and lack of childcare and acquiring food to take with medication made daily adherence remain an ongoing issue. These findings are consistent with previous studies that demonstrate that transportation affordability and food insecurity may inadvertently lead to disengagement in care and poor viral suppression. 39 , 40 , 41 To address this, integrated interventions with transport reimbursement 42 and decentralization of clinic services into community‐based services 43 have been successful in improving HIV programme outcomes. Providing food supplements and nutritional support to ART patients as a public health service also may improve adherence. 44 , 45 , 46
Government disability social grants provide financial assistance to persons with chronic illnesses such as HIV. However, with eligibility requirements of poor HIV indicators, such as high viral loads, individuals may feel disincentivized to improve their health with the fear of losing their only source of income. 47 The termination of disability grants when HIV indicators improve should be reviewed and, at the very least, transitioning of individuals into a nutritional and/or transport reimbursement programme may be beneficial.
Women did not feel safe to travel to and from the clinic because of gang violence and increased criminal activity related to having their ART medication stolen. Several studies in South Africa have characterized the use of HIV medication with illicit substances, raising concerns that antiretroviral abuse jeopardizes the safety of both patients and drug users. 48 , 49 , 50 The emerging recreational use of ART, diversion of ART to others through violence and the associated risks of antiretroviral resistance and violence should be at the forefront of community health policy concerns.
Stigma also was attributed to disengagement in care. People living with HIV who reported high levels of stigma are over four times more likely to report poor access to care. 51 Additionally, HIV‐related stigma has downstream effects on HIV morbidity, mortality and quality of life for people living with HIV. 21 , 52 Fear of unintended HIV status disclosure and its repercussion when attending clinic appointments deters health‐seeking behaviour. Often people living with HIV fear attending local community healthcare facilities and will sometimes choose to attend clinics that are far from their local facilities to avoid being recognized. 53 , 54 Also, harsh treatment from clinic staff and stigmatizing language often lead to a feeling of moral failing and shame, resulting in treatment interruption. Incorporating facility‐wide stigma‐reduction strategies in staff training and health programmes would foster improved patient–clinic staff relationships. 55 , 56 Experiences of stigma may lead people living with HIV to be too afraid to receive much‐needed support from their family members. Normative community stigmatizing beliefs and attitudes related to HIV (community‐level HIV stigma) can negatively impact health‐seeking behaviour among people living with HIV. 57 Responding to stigma at multiple levels is critical to achieving global targets for HIV testing, linkage to care and viral suppression. 1
Health service structural barriers, such as health system logistical issues and lack of synergy and integration of health services, increase long wait times, cause delays and have been found to reduce clinic attendance. 58 Staffing and time constraints may lead to inadequate counselling and lack of rapport building with individuals as they initiate ART. 59 Healthcare staff also have reported that HIV infection is so prevalent in South Africa that at times it is normalized, desensitizing staff to the extent of education needed by newly diagnosed individuals. 18
These findings point to the overlapping socioecological barriers related to the treatment journey among women living with HIV who use AODs. It is important to note that despite the barriers to adherence mentioned, all participants reported that being part of the WHC facilitated their attempts to adhere to their medication through social support for AOD use and ART adherence and encouragement from fellow participants. 22 However, in order for this successful gender‐focused intervention to sustain positive outcomes, it should be coupled with interventions that address broader contextual factors that influence retention in HIV care across multiple domains of women living with HIV with comorbidities such as substance use.
4.1. Limitations and strengths
Participants' perspectives and experiences may have been restricted to recall bias. To reduce the likelihood of this bias, focus group discussions were conducted by staff who did not conduct the WHC workshops. The back‐and‐forth translations during the focus groups may have affected the validity of the focus groups’ data. However, these issues were addressed with real‐time interpreters who understood the questions, the social context, cultural background and language spoken by the participants. However, the strengths of this study include the inclusion of a key population that is difficult to reach and is likely to disengage in HIV care. Additionally, the study findings provide validation with qualitative data from healthcare providers regarding barriers to ART adherence among this same population of women living with HIV. 60
5. CONCLUSION
The perspectives of the study participants provided meaningful insight into the external barriers to care among women who had successfully completed a behaviour change intervention emphasizing the importance of understanding the context in which a behaviour takes place. The study findings reflect the broad effects of socioecological barriers to ART adherence that should be addressed in tandem. Foremost, healthcare providers are in a unique position to create a person‐centred experience where ART fits in a patient's complex environment. With the rollout of the universal test‐and‐treat approach, there is a need for further assessment of an individual's ability to initiate and adhere to ART based on underlying challenges, such as AOD use and other social issues. Additionally, continuous booster sessions of brief, effective, gender‐focused interventions, such as the WHC, including screening, counselling and referrals for AOD use integrated into HIV care, may result in longer‐term improvements in ART adherence. Furthermore, as with the WHC, it may be beneficial to integrate community‐based, gender‐focused, support groups and other forms of differentiated care models that provide a safe nondiscriminatory space for patients who have disengaged in care. The goal of these groups would be to provide gender‐specific support in addressing and overcoming barriers to ART adherence; and when stable, individuals could graduate to the conventional ART adherence club or remain to support their peers. Social determinants of health, such as economic stability, affordable housing and transportation, food security and healthcare access have a significant influence on health outcomes. With ART being a lifelong healthcare commitment, comprehensive health services support along the course of the HIV care continuum and government policies that improve the living conditions of people living with HIV will be essential to achieving the 95‐95‐95 UNAIDS targets by 2030. Finally, these recommendations cannot be successful without addressing the drivers and facilitators of multiple‐level stigma, a major barrier to health‐seeking behaviour.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHORS CONTRIBUTIONS
Jacqueline W. Ndirangu conducted the interviews, interpreted the data, drafted and finalized the manuscript. Wendee M. Wechsberg conceived and designed the study and finalized the manuscript. Margaret W. Gichane and Erin N. Cox coded and analysed the qualitative data. Felicia A. Browne, Courtney P. Bonner, William A. Zule, critically reviewed and revised the manuscript. Kevin M. Smith contributed to the Background section of the manuscript. Tara Carney assisted in conducting the interviews. All authors reviewed, read and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank Brittni Howard and Jeffrey Novey for his editorial support, in addition to the research staff for their contributions to the study and the research participants for their involvement in the study. This study was conducted with support from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) under grant R01AA022882 (PI: Wendee M. Wechsberg). The findings and conclusions presented in this article are those of the authors and do not necessarily represent the views of the NIAAA. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ndirangu JW, Gichane MW, Browne FA, et al. ‘We have goals but [it is difficult]’. Barriers to antiretroviral therapy adherence among women using alcohol other drugs living with HIV in South Africa. Health Expect. 2022;25:754‐763. 10.1111/hex.13422
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restriction.
REFERENCES
- 1. UNAIDS . UNAIDS data. 2019. Accessed September 20, 2020. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf
- 2. UNAIDS Prevention gap report. 2016. Accessed March 8, 2021. https://www.unaids.org/en/resources/documents/2016/prevention-gap
- 3. Simbayi L, Zuma K & Zungu N, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: towards achieving the UNAIDS 90-90-90 targets. HSRC Press; 2019.
- 4. Kader R, Govender R, Seedat S, Koch JR, Parry C. Understanding the impact of hazardous and harmful use of alcohol and/or other drugs on ARV adherence and disease progression. PLoS One. 2015;10(5):e0125088. 10.1371/journal.pone.0125088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalichman S, Mathews C, Banas E, Kalichman M. Alcohol‐related intentional nonadherence to antiretroviral therapy among people living with HIV, Cape Town, South Africa. AIDS Care. 2019;31(8):951‐957. 10.1080/09540121.2019.1587357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luseno WK, Wechsberg WM, Kline TL, Ellerson RM. Health services utilization among South African women living with HIV and reporting sexual and substance‐use risk behaviors. AIDS Patient Care STDS. 2010;24(4):257‐264. 10.1089/apc.2009.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul Health Metr. 2017;15(1):4. 10.1186/s12963-017-0121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wechsberg WM, Luseno W, Riehman K, Karg R, Browne F, Parry C. Substance use and sexual risk within the context of gender inequality in South Africa. Subst Use Misuse. 2008;43(8‐9):1186‐1201. 10.1080/10826080801918247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wechsberg WM, Jewkes R, Novak SP, et al. A brief intervention for drug use, sexual risk behaviours and violence prevention with vulnerable women in South Africa: a randomised trial of the Women's Health CoOp. BMJ Open. 2013;3(5):e002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wechsberg WM, Ndirangu JW, Speizer IS, et al. An implementation science protocol of the Women's Health CoOp in healthcare settings in Cape Town, South Africa: a stepped‐wedge design. BMC Womens Health. 2017;17(1):85. 10.1186/s12905-017-0433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059‐2067. 10.1097/QAD.0b013e3283578b9b [DOI] [PubMed] [Google Scholar]
- 12. Scott‐Sheldon LA, Carey KB, Johnson BT, Carey MP, MASH Research Team . Behavioral interventions targeting alcohol use among people living with HIV/AIDS: a systematic review and meta‐analysis. AIDS Behav. 2017;21(2):126‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrasco MA, Esser MB, Sparks A, Kaufman MR. HIV‐alcohol risk reduction interventions in Sub‐Saharan Africa: a systematic review of the literature and recommendations for a way forward. AIDS Behav. 2016;20(3):484‐503. 10.1007/s10461-015-1233-5 [DOI] [PubMed] [Google Scholar]
- 14. Wechsberg WM, Bonner CP, Zule WA, et al. Addressing the nexus of risk: biobehavioral outcomes from a cluster randomized trial of the Women's Health CoOp Plus in Pretoria, South Africa. Drug Alcohol Depend. 2019;195:16‐26. 10.1016/j.drugalcdep.2018.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wechsberg WM, Zule WA, Luseno WK, et al. Effectiveness of an adapted evidence‐based woman‐focused intervention for sex workers and non‐sex workers: the Women's Health CoOp in South Africa. J Drug Issues. 2011;41(2):233‐252. 10.1177/002204261104100205 [DOI] [Google Scholar]
- 16. Becker N, Cordeiro LS, Poudel KC, Sibiya TE, Sayer AG, Sibeko LN. Individual, household, and community level barriers to ART adherence among women in rural Eswatini. PLoS One. 2020;15(4):e0231952. 10.1371/journal.pone.0231952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: challenges and potential ways forward. Glob Public Health. 2011;6(1):83‐97. 10.1080/17441691003796387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loeliger KB, Niccolai LM, Mtungwa LN, Moll A, Shenoi SV. Antiretroviral therapy initiation and adherence in rural South Africa: community health workers' perspectives on barriers and facilitators. AIDS Care. 2016;28(8):982‐993. 10.1080/09540121.2016.1164292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adimora AA, Auerbach JD. Structural interventions for HIV prevention in the United States. J Acquir Immune Defic Syndr. 2010;55(suppl 2, 02):S132‐S135. 10.1097/QAI.0b013e3181fbcb38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008;372(9640):764‐775. 10.1016/s0140-6736(08)60887-9 [DOI] [PubMed] [Google Scholar]
- 21. Logie CH, Wang Y, Lacombe‐Duncan A, et al. HIV‐related stigma, racial discrimination, and gender discrimination: pathways to physical and mental health‐related quality of life among a national cohort of women living with HIV. Prev Med. 2018;107:36‐44. 10.1016/j.ypmed.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 22. Wechsberg WM, Browne FA, Ndirangu J, et al. Outcomes of implementing in the real world the Women's Health CoOp Intervention in Cape Town, South Africa. AIDS Behav. 2021;25:276‐289. 10.1007/s10461-021-03251-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guest G, MacQueen KM, Namey EE. Introduction to applied thematic analysis. Applied Thematic Analysis. Vol 3, 2012:20. [Google Scholar]
- 24. Dewey ME. Coefficients of agreement. Br J Psychiatry. 1983;143(5):487‐489. [DOI] [PubMed] [Google Scholar]
- 25. Davis A, Pala AN, Nguyen N, et al. Sociodemographic and psychosocial predictors of longitudinal antiretroviral therapy (ART) adherence among first‐time ART initiators in Cape Town, South Africa. AIDS Care. 2021;33(11):1394‐1403. 10.1080/09540121.2020.1798336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cichowitz C, Maraba N, Hamilton R, Charalambous S, Hoffmann CJ. Depression and alcohol use disorder at antiretroviral therapy initiation led to disengagement from care in South Africa. PLoS One. 2017;12(12):e0189820. 10.1371/journal.pone.0189820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fatch R, Emenyonu NI, Muyindike W, Kekibiina A, Woolf‐King S, Hahn JA. Alcohol interactive toxicity beliefs and ART non‐adherence among HIV‐infected current drinkers in Mbarara, Uganda. AIDS Behav. 2017;21(7):1812‐1824. 10.1007/s10461-016-1429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalichman SC, Amaral CM, White D, et al. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care. 2012;23(6):511‐520. 10.1016/j.jana.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sundararajan R, Wyatt MA, Woolf‐King S, et al. Qualitative study of changes in alcohol use among HIV‐infected adults entering care and treatment for HIV/AIDS in rural southwest Uganda. AIDS Behav. 2015;19(4):732‐741. 10.1007/s10461-014-0918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. HIVinfo.NIH.gov. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. NIH Office of AIDS Research. Updated June 3, 2021. Accessed June 10, 2021. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/substance-use-disorders-and-hiv
- 31. Durvasula R, Miller TR. Substance abuse treatment in persons with HIV/AIDS: challenges in managing triple diagnosis. Behav Med. 2014;40(2):43‐52. 10.1080/08964289.2013.866540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalichman SC, Kalichman MO, Cherry C. Forget about forgetting: structural barriers and severe non‐adherence to antiretroviral therapy. AIDS Care. 2017;29(4):418‐422. 10.1080/09540121.2016.1220478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cornelius T, Jones M, Merly C, Welles B, Kalichman MO, Kalichman SC. Impact of food, housing, and transportation insecurity on ART adherence: a hierarchical resources approach. AIDS Care. 2017;29(4):449‐457. 10.1080/09540121.2016.1258451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walcott M, Kempf M‐C, Merlin JS, Turan JM. Structural community factors and sub‐optimal engagement in HIV care among low‐income women in the Deep South of the USA. Cult Health Sex. 2016;18(6):682‐694. 10.1080/13691058.2015.1110255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bepouka B, Situakibanza H, Kiazayawoko F, Aliocha N. P3.23 Determinants of non‐adherence to antiretroviral therapy in adults in the city of Kinshasa. Sex Transm Infect. 2017;93(suppl 2):A101‐A102. 10.1136/sextrans-2017-053264.260 [DOI] [Google Scholar]
- 36. Musumari PM, Feldman MD, Techasrivichien T, Wouters E, Ono‐Kihara M, Kihara M. “If I have nothing to eat, I get angry and push the pills bottle away from me”: a qualitative study of patient determinants of adherence to antiretroviral therapy in the Democratic Republic of Congo. AIDS Care. 2013;25(10):1271‐1277. 10.1080/09540121.2013.764391 [DOI] [PubMed] [Google Scholar]
- 37. Pellowski JA, Huedo‐Medina TB, Kalichman SC. Food insecurity, substance use, and sexual transmission risk behavior among people living with HIV: a daily level analysis. Arch Sex Behav. 2018;47(7):1899‐1907. 10.1007/s10508-017-0942-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Young S, Wheeler AC, McCoy SI, Weiser SD. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav. 2014;18(suppl 5, 05):S505‐S515. 10.1007/s10461-013-0547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Igumbor JO, Scheepers E, Ebrahim R, Jason A, Grimwood A. An evaluation of the impact of a community‐based adherence support programme on ART outcomes in selected government HIV treatment sites in South Africa. AIDS Care. 2011;23(2):231‐236. 10.1080/09540121.2010.498909 [DOI] [PubMed] [Google Scholar]
- 40. Kalichman SC, Washington C, Grebler T, et al. Medication adherence and health outcomes of people living with HIV who are food insecure and prescribed antiretrovirals that should be taken with food. Infect Dis Ther. 2015;4(1):79‐91. 10.1007/s40121-015-0057-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singer AW, Weiser SD, McCoy SI. Does food insecurity undermine adherence to antiretroviral therapy? A systematic review. AIDS Behav. 2015;19(8):1510‐1526. 10.1007/s10461-014-0873-1 [DOI] [PubMed] [Google Scholar]
- 42. Siedner MJ, Santorino D, Lankowski AJ, et al. A combination SMS and transportation reimbursement intervention to improve HIV care following abnormal CD4 test results in rural Uganda: a prospective observational cohort study. BMC Med. 2015;13:160. 10.1186/s12916-015-0397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bemelmans M, van den Akker T, Ford N, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15(12):1413‐1420. 10.1111/j.1365-3156.2010.02649.x [DOI] [PubMed] [Google Scholar]
- 44. Cantrell RA, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food‐insecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;49(2):190‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCoy SI, Njau PF, Fahey C, et al. Cash vs. food assistance to improve adherence to antiretroviral therapy among HIV‐infected adults in Tanzania. AIDS. 2017;31(6):815‐825. 10.1097/QAD.0000000000001406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tirivayi N, Koethe JR, Groot W. Clinic‐based food assistance is associated with increased medication adherence among HIV‐infected adults on long‐term antiretroviral therapy in Zambia. J AIDS Clin Res. 2012;3(7):171. 10.4172/2155-6113.1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Govender V, Fried J, Birch S, Chimbindi N, Cleary S. Disability grant: a precarious lifeline for HIV/AIDS patients in South Africa. BMC Health Serv Res. 2015;15(1):227. 10.1186/s12913-015-0870-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grelotti DJ, Closson EF, Smit JA, et al. Whoonga: potential recreational use of HIV antiretroviral medication in South Africa. AIDS Behav. 2014;18(3):511‐518. 10.1007/s10461-013-0575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuo C, Giovenco D, DeAtley T, et al. Recreational use of HIV antiretroviral medication and implications for HIV pre‐exposure prophylaxis and treatment. AIDS Behav. 2020;24(9):2650‐2655. 10.1007/s10461-020-02821-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rough K, Dietrich J, Essien T, et al. Whoonga and the abuse and diversion of antiretrovirals in Soweto, South Africa. AIDS Behav. 2014;18(7):1378‐1380. 10.1007/s10461-013-0683-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self‐reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101‐1108. 10.1007/s11606-009-1068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stangl AL, Earnshaw VA, Logie CH, et al. The Health Stigma and Discrimination Framework: a global, crosscutting framework to inform research, intervention development, and policy on health‐related stigmas. BMC Med. 2019;17(1):31. 10.1186/s12916-019-1271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nzaumvila DK, Mabuza LH. Why do women not return for CD4 count results at Embhuleni Hospital, Mpumalanga, South Africa? Curationis. 2015;38(1), 1266. 10.4102/curationis.v38i1.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Treves‐Kagan S, Steward WT, Ntswane L, et al. Why increasing availability of ART is not enough: a rapid, community‐based study on how HIV‐related stigma impacts engagement to care in rural South Africa. BMC Public Health. 2016;16(1):87. 10.1186/s12889-016-2753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nyblade L, Addo NA, Atuahene K, et al. Results from a difference‐in‐differences evaluation of health facility HIV and key population stigma‐reduction interventions in Ghana. J Int AIDS Soc. 2020;23(4):e25483. 10.1002/jia2.25483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nyblade L, Stockton MA, Giger K, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med. 2019;17(1):25. 10.1186/s12916-019-1256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Daniel Kelly J, Reid MJ, Lahiff M, Tsai AC, Weiser SD. Community‐level HIV stigma as a driver for HIV transmission risk behaviors and sexually transmitted diseases in Sierra Leone: a population‐based study. J Acquir Immune Defic Syndr. 2017;75(4):399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mwamba C, Sharma A, Mukamba N, et al. ‘They care rudely!’: resourcing and relational health system factors that influence retention in care for people living with HIV in Zambia. BMJ Global Health. 2018;3:e001007. [DOI] [PMC free article] [PubMed]
- 59. Agarwal R, Rewari BB, Allam RR, Chava N, Rathore AS. Quality and effectiveness of counselling at antiretroviral therapy centres in India: capturing counsellor and beneficiary perspectives. Int Health. 2019;11(6):480‐486. 10.1093/inthealth/ihy100 [DOI] [PubMed] [Google Scholar]
- 60. Gichane MW, Wechsberg WM, Ndirangu J, et al. Implementation science outcomes of a gender‐focused HIV and alcohol risk‐reduction intervention in usual‐care settings in South Africa. Drug Alcohol Depend. 2020;215:108206. 10.1016/j.drugalcdep.2020.108206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restriction.


