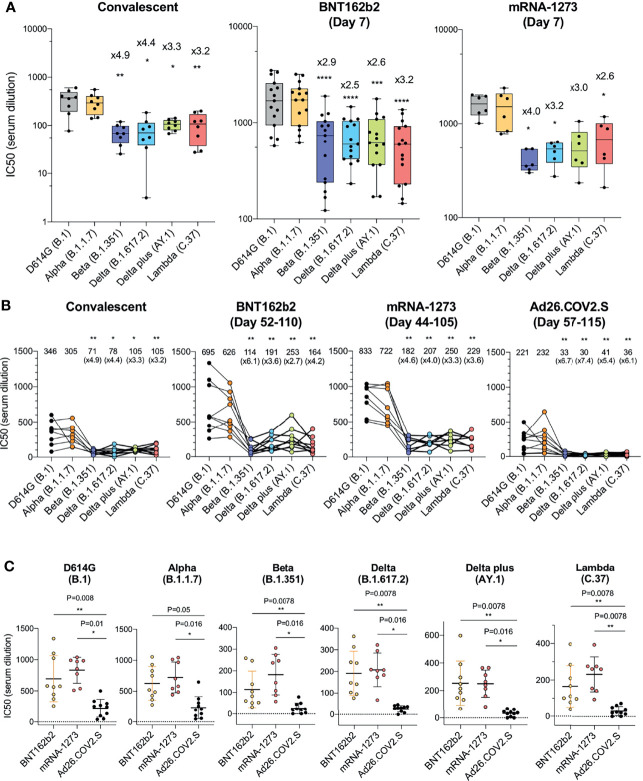
Figure 1.
Comparison of neutralization titers of variant spike protein pseudotyped viruses by convalescent sera, antibodies elicited by BNT162b2, mRNA-1273, Ad26.COV2.S. (A) Neutralization of variant spike protein pseudotyped viruses by convalescent serum (n = 8) (left). Neutralizing titers of serum samples from BNT162b2 vaccinated individuals (n = 15) (middle). Neutralizing titers of serum samples from mRNA-1273 vaccinated donors (n = 6) (right). The serum was collected at early time point (7 days after second immunization). Serum samples from Ad26.COV2.S vaccinated donors were not available. The neutralization IC50 from individual donors is shown. Significance is based on two-sided t-test. Units on the Y axis of the graph for convalescent serum samples has been adjusted to allow for visualization of the data. (B) Comparison of neutralization of variants by convalescent serum (n = 8, the same donors in A), BNT162b2 vaccinated individuals (n = 9), mRNA-1273 vaccinated donors (n = 8), Ad26.COV2.S vaccinated donors (n = 10), sera from vaccinated individuals were collected at later time points [Day 52-110 (mean 90), Day 44-105 (mean 80), Day 57-115 (mean 82 days) after last immunization of each vaccine, see the Supplementary Table 2]. Each line shows individual donors. (C) Comparison of neutralization potency of each vaccine by different SARS-CoV-2 variants. The neutralization IC50 from individual donors vaccinated by BNT162b2 (yellow), mRNA-1273 (pink), Ad26.COV2.S (black) is shown. Significance is based on two-sided t-test. (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).