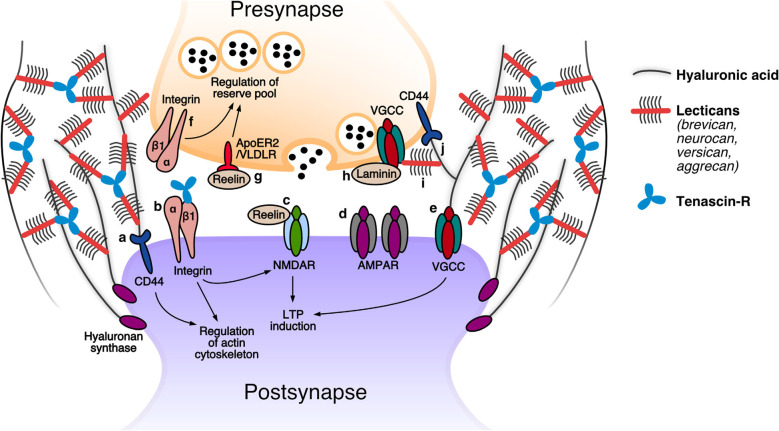
Figure 1.
The tetrapartite synapse: speculated roles of extracellular matrix (ECM) molecules. Postsynapse: (a) The receptor for hyaluronan CD44 was demonstrated to modulate the activity of actin-associated proteins and thereby promote the stabilization of dendritic spines (Roszkowska et al., 2016). (b) β1 integrin receptors are proposed to contribute to LTP by modulating NMDAR-mediated currents, and play a role in spine restructuring and stabilization by modulating the actin cytoskeleton. An interaction has been reported between β1 integrins and tenascin-R (TNR) (Bernard-Trifilo et al., 2005; Liao et al., 2008; Tan et al., 2011; Sloan Warren et al., 2012). (c) Increased levels of reelin augment NMDAR-mediated currents, leading to enhanced LTP responses (Weeber et al., 2002; Rogers et al., 2011). (d) The hyaluronan-based perisynaptic ECM restricts the lateral mobility of AMPARs (Frischknecht et al., 2009). (e) Hyaluronan regulates VGCC-dependent plasticity by modulating these channels (Kochlamazashvili et al., 2010). Presynapse: (f) Presynaptic β1 integrin receptors are presumed to regulate the reserve pool of synaptic vesicles (Huang et al., 2006). (g) Reelin activation of its ApoER2 and VLDLR receptors modulates reserve pool synaptic vesicles (Bal et al., 2013). (h) Presynaptic active zone proteins are anchored at the membrane through putative interactions with β2 laminins (such as with VGCCs; Nishimune et al., 2004; Hunter et al., 2019). (i) The presence of perisynaptic brevican is essential for a correct alignment of presynaptic VGCCs in front of the postsynaptic density (Sonntag et al., 2018). (j) CD44, the receptor for hyaluronan, was shown to be present presynaptically and is essential for presynapse stability (Roszkowska et al., 2016). Modified from Dankovich (2021).