FIGURE 1.
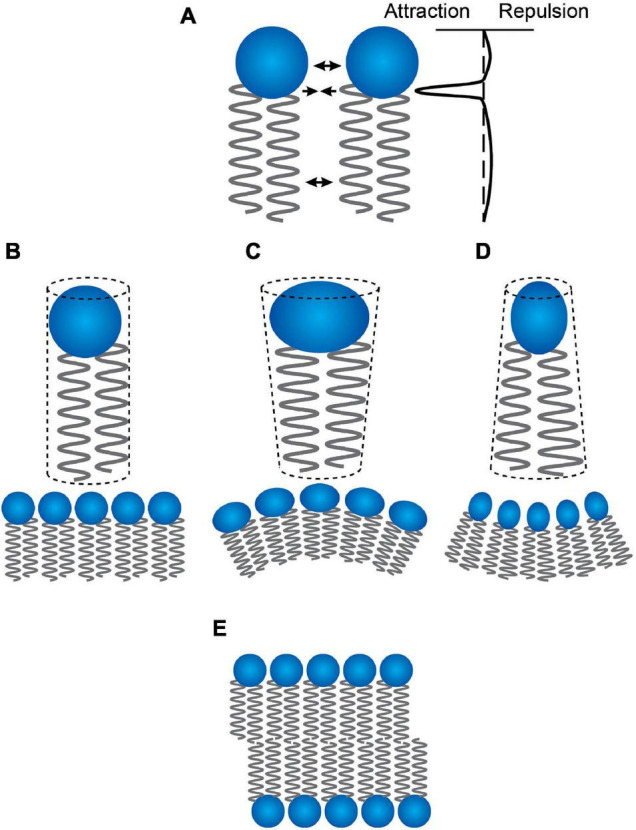
Lipid “shape” and curvature. (A) the profile of intermolecular interactions between two lipid molecules in a bilayer. Changes in this profile alter the effective lipid “shape” and the intrinsic curvature, c0, of the bilayer-forming lipids. (B) Lipids that have a cylindrical shape form plane monolayers with no intrinsic curvature. (C) Increased repulsion between the head groups will cause the lipids to be cone-shaped with the broad base toward the aqueous solution—and to form monolayers with a positive curvature (the surface is convex when viewed from the aqueous solution). (D) Decreased repulsion between the head groups will cause the lipids to be cone-shaped with the broad base toward the terminal methyl groups—and to form monolayers with a negative curvature (the surface is concave when viewed from the aqueous solution). (E) As long as the curvature is not too extreme, all three types of lipids can form planar bilayers—with the bilayers formed by lipids having intrinsic curvature being under a curvature frustration stress, which will contribute the energetics of channel formation (the energetic cost of a channel-imposed bilayer thinning will decrease as c0 increases and increase as c0 decreases).
