FIGURE 2.
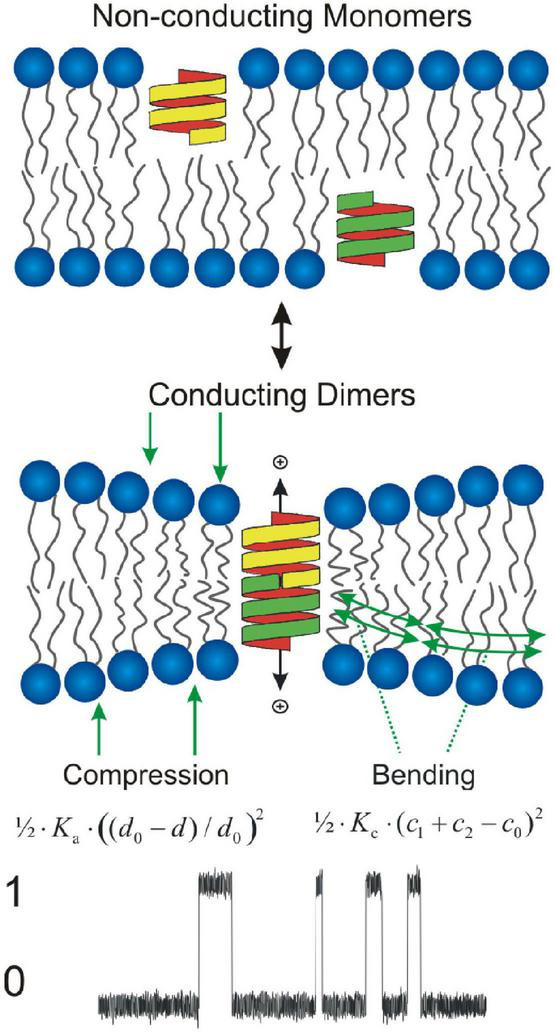
Gramicidin channels as molecular force probes. Gramicidin channels form by transmembrane association of b6⋅3-helical monomers imbedded in each monolayer, which is associated with compression and bending of the two bilayer leaflets toward each other. The local bilayer deformation imposed by the hydrophobic mismatch between the channel and its host bilayer, produces a compression of (each leaflet of) the bilayer with an energy density 1/2⋅Ka⋅((d0−d)/d0)2, where Ka denotes the elastic area-compression modulus, d0 the thickness of the unperturbed bilayer, and d the local bilayer thickness as function of distance from the channel, and a bending of the each monolayer/solution interface with an energy density we approximate as 1/2⋅Kc⋅(c1 + c2−c0)2 where c1 and c2 denote the principal components of the local curvature. Bottom: Single channel current transitions associated with channel formation/dissociation (0: no channel; 1: conducting channel).
