To the Editor: Few studies have directly investigated the effects of psoriatic disease and the use of immunomodulatory therapies on COVID-19 outcomes. Here, we report the COVID-19 outcomes of patients with psoriatic disease from a multidisciplinary, prospective, cohort study, namely, Web-based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic.1
Overall, 173 adults with psoriasis and/or psoriatic arthritis were recruited from NYU Langone Health (IRB#i20-00389) and NYC Health + Hospitals/Bellevue (IRB#STUDY00002387) and followed from March to July 2020. The participants were referred by a dermatologist or rheumatologist or had previously consented to be contacted for research purposes. Pairwise t-test and Fisher’s exact/χ2 test were used to compare demographic and clinical characteristics. Adjusted logistic regression models were used to assess the effect of comorbidities and immunomodulatory therapies on COVID-19 outcomes. Statistical analyses were conducted using R, version 3.6.1.
Fifty-eight (33.5%) patients (40 confirmed; 18 high suspicion) contracted COVID-19. Most patients (81%) who developed COVID-19 had mild disease (managed at home or in an outpatient setting). Eleven patients (19%) were hospitalized for severe disease, including 4 (6.9%) who were treated on the floor and 6 (10%) who required intensive care. Four patients (6.9%) died from COVID-19-related complications. All 4 patients had preexisting risk factors for severe infection, and 2 had high-risk exposures to a healthcare setting (eg, recent hospital visits or occupational exposures to hospital). There was no significant difference in age, sex, or underlying psoriatic disease among the controls, cases, or those who had severe COVID-19 (Table I).
Table I.
Characteristics of COVID-19 cases and controls in patients with psoriatic disease
| Patient characteristic | COVID-19 cases N=58 |
Patients with severe COVID-19 N=11 |
Controls N = 115 |
P value§ |
|---|---|---|---|---|
| Age | 52.8 | 60.6 | 57.1 | .058∗ |
| Gender (N, % Female) | 30 (51.7%) | 7 (9.1%) | 63 (54.8%) | .826∗ |
| BMI | 29.7 | 34.5 | 26.4 | .010∗,† |
| Race (N, %) | .046∗,† | |||
| White | 44 (75.9%) | 7 (63.6%) | 104 (91.3%) | |
| Black | 1 (1.7%) | 1 (9.1%) | 1 (0.9%) | |
| Asian | 8 (13.8%) | 3 (27.3%) | 6 (5.2%) | |
| Other | 4 (6.9%) | 0 (0%) | 1 (0.9%) | |
| Psoriasis (N, %) | 35 (65.5%) | 86 (75.8%) | .075∗ | |
| Psoriatic arthritis (Number, %) | 42 (72.4%) | 8 (72.7%) | 78 (67.8%) | .658∗ |
| Severity of psoriatic disease | .641∗ | |||
| Remission/Mild (%) | 11 (19.0%) | 1 (9.1%) | 15 (13.0%) | |
| Mild | 25 (43.1%) | 4 (36.4%) | 53 (46.1%) | |
| Moderate | 17 (29.3%) | 4 (36.4%) | 40 (34.9%) | |
| Severe | 5 (8.6%) | 2 (18.2%) | 7 (6.1%) | |
| Psoriatic disease therapy (Number, %) | ||||
| Methotrexate | 11 (19.0%) | 2 (18.2%) | 25 (21.7%) | .8213∗ |
| Oral glucocorticoids | 2 (3.4%) | 0 (0%) | 4 (3.5%) | .99∗ |
| Apremilast | 3 (5.2%) | 1 (9.1%) | 8 (7.0%) | .731∗ |
| Any biologic or JAK inhibitor | 42 (72.4%) | 7 (63.6%) | 65 (56.5%) | .062∗ |
| TNF-inhibitors | 13 (22.4%) | 4 (36.4%) | 29 (25.2%) | 1.00∗ |
| IL-17 blockers | 15 (25.9%) | 2 (18.2%) | 23 (20.0%) | .288∗ |
| IL-12/23 or IL-23 blockers | 11 (19.0%) | 1 (9.1%) | 7 (6.1%) | .008∗,† |
| JAK inhibitors | 1 (1.7%) | 1 (9.1%) | 6 (5.2%) | .426∗ |
| Comorbidity | ||||
| CHF (N, %) | 4 (6.9%) | 1 (9.1%) | 0 (0%) | N/A‡ |
| HTN | 11 (19.0%) | 4 (36.4%) | 7 (6.1%) | .019∗,† |
| DM2 | 13 (11.3%) | 2 (18.2%) | 8 (13.8%) | .821∗ |
| COPD§ | 2 (1.7%) | 1 (9.1%) | 2 (3.4%) | .6027∗ |
| Asthma | 10 (8.7%) | 1 (9.1%) | 9 (15.5%) | .2726∗ |
| CKD | 1 (1.6%) | 0 (0%) | 1 (0.9%) | 1.00∗ |
| Liver disease | 2 (3.4%) | 0 (0%) | 0 (0%) | N/A‡ |
| Obesity | 19 (32.8%) | 5 (45.5%) | 25 (21.7%) | .1657∗ |
BMI, Body mass index; CHF, congestive heart faliure; CKD; chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM2, type 2 diabetes mellitus; HTN, hypertension; IL, interleukin; JAK, janus kinase; TNF, tumor necrosis factor.
P value for all COVID-19 cases vs. controls.
P value for severe COVID-19 vs. controls or mild COVID-19.
No P values can be calculated when the category has 0 events.
Reported P values are not adjusted for multiple comparisons.
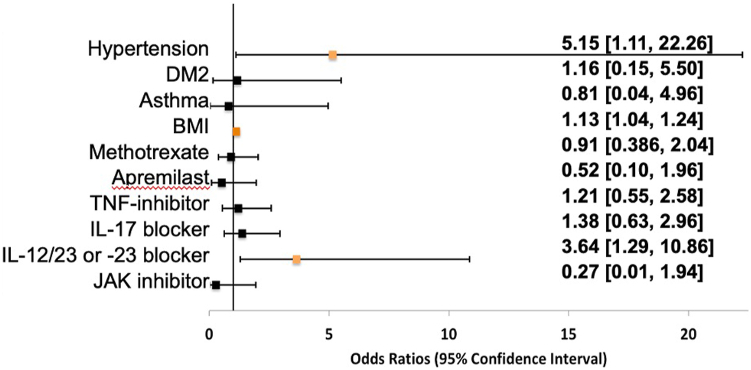
Hypertension (odds ratio [OR] = 5.5; 95% confidence interval [CI] = 1.11-22.26; P = .028) and high body mass index (BMI) (OR = 1.13; CI = 1.04-1.24; P = .005) were associated with severe COVID-19 outcomes (Fig 1). Interleukin 12 (IL-12)/interleukin 23 (IL-23) or IL-23 inhibitor therapy was associated with an increased risk of contracting COVID-19 (OR = 3.64; CI = 1.29-10.86; P = .016) but not with an increased risk of developing more severe disease. The use of methotrexate, oral glucocorticoids, apremilast, tumor necrosis factor-alpha inhibitors, and interleukin 17 inhibitors did not affect COVID-19 outcomes.
Fig 1.
Logistic regression analysis of the effects of comorbidities (adjusted for age, sex, and psoriasis disease severity) on the risk of developing severe COVID-19 in patients with psoriatic disease and of the effects of psoriatic disease therapies (adjusted for age, sex, and BMI) on the risk of developing COVID-19 in patients with psoriatic disease. Odds ratios are not adjusted for multiple comparisons. BMI, Body mass index; COVID-19, coronavirus disease 2019; DM2, type 2 diabetes mellitus; IL, interleukin; JAK, janus kinase;TNF, tumor necrosis factor.
The hospitalization rate in our cohort was similar to that of the general New York City population at the time of data capture (21%),2 suggesting that psoriatic disease alone does not confer a higher risk of developing severe COVID-19. The association among hypertension, elevated BMI, and more severe outcomes in COVID-19 is consistent with data from a European psoriasis cohort.3 Patient counseling on these risk factors may be important as psoriatic disease is associated with cardiometabolic comorbidities.
Because of the low incidence of COVID-19, referred cases were needed to obtain an adequate number for meaningful statistical analysis, and this may have led to selection bias. Therefore, the association between IL-12/23 or IL-23 blocker use and SARS-CoV-2 infection should be interpreted with caution. Interestingly, an Italian study also found an association between biologic therapy and COVID-19 but not an increased risk of severe outcomes.4 Further investigation is needed to understand the effects of biologics on SARS-CoV-2 infection. The National Psoriasis Foundation task force has suggested that biologic therapies do not meaningfully alter the risk of developing COVID-19 and should be continued in the absence of infection.5
Conflicts of interest
None declared.
Footnotes
IRB approval status: This study was approved by the NYU Langone Health (IRB#i20-00389) and NYC Health + Hospitals/Bellevue (IRB#STUDY00002387) IRB boards.
Funding sources: Supported by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 2020 Pilot Research Grant (DY and RC).
References
- 1.Haberman R.H., Castillo R., Chen A., et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol. 2020;72(12):1981–1989. doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health NYC. COVID-19: Data. City of New York. May 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data-archive.page
- 3.Lima X.T., Cueva M.A., Lopes E.M., Alora M.B. Severe COVID-19 outcomes in patients with psoriasis. J Eur Acad Dermatol Venereol. 2020;34(12):e776–e778. doi: 10.1111/jdv.16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damiani G., Pacifico A., Bragazzi N.L., Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33(5):e13475. doi: 10.1111/dth.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand J.M., Armstrong A.W., Bell S., et al. National psoriasis foundation COVID-19 task force guidance for management of psoriatic disease during the pandemic: version 2-advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. 2021;84(5):1254–1268. doi: 10.1016/j.jaad.2020.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]