Abstract
Objectives
The purpose of this study was to compare the efficacy and safety of drug-eluting beads transarterial chemoembolization plus camrelizumab (D-TACE-C) with conventional transarterial chemoembolization plus camrelizumab (C-TACE-C) in the treatment of patients with unresectable hepatocellular carcinoma (HCC).
Materials and Methods
This was a retrospective study that evaluated the consecutive medical records of patients with unresectable HCC who had undergone D-TACE-C or C-TACE-C from April 2020 to August 2021. Efficacy of treatment was evaluated using tumor response, progression-free survival (PFS) and survival rates. The adverse events were recorded.
Results
A total of 54 patients were included in this study, including 27 patients who had received D-TACE-C treatment, and 27 patients who had received C-TACE-C treatment. The median PFS and DCR in the D-TACE-C group were significantly longer than those for the C-TACE-C group (PFS: 10 vs. 3 months, P=.017; DCR: 70.4% vs. 40.7%, P = .028). Cox regression analysis showed that D-TACE-C was the only protective factor for PFS. The 6-month and 12-month survival rates in D-TACE-C group and C-TACE-C group were 85.2% versus 79.4% (P = .646) and 65.2% versus 65.1% (P = .903), respectively. Reactive cutaneous capillary endothelial proliferation was the most common adverse event associated with the treatment. There was no significant difference in the adverse events related to TACE and camrelizumab between the two groups. No treatment-related deaths occurred in this study.
Conclusions
D-TACE-C is a safe and well-tolerated treatment, and may produce better PFS and tumor response in patients with unresectable HCC than C-TACE-C.
Keywords: camrelizumab, drug-eluting beads transarterial chemoembolization, progression-free survival, hepatocellular carcinoma, combination therapy, efficacy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common carcinoma and the second leading cause of tumor-related death. 1 Most patients are first diagnosed when the disease is at the intermediate or advanced stage due to its insidious onset and rapid progress. This limits the number of patients who can benefit from curative treatments such as resection, liver transplantation, and radiofrequency ablation since they are only suitable for patients at early stages. 2
Currently, transarterial chemoembolization (TACE), which combines targeted chemotherapy with ischemic necrosis caused by arterial embolization, is recommended as the first-treatment for HCC patients at BCLC stage B. 3 In conventional TACE (C-TACE), lipiodol acts as an embolic agent and carrier of chemotherapeutic drugs (such as doxorubicin) to the tumor. The doxorubicin is released slowly near the target nodules, resulting in a combination of sustained chemotherapeutic effects and tumor ischemia. 4 However, C-TACE has important technical and scheduling drawbacks that have not yet been standardized. 5 As a result, lipiodol releases doxorubicin in an unsustained manner and side effects occur due to higher systemic concentrations of doxorubicin.6,7
The drug-eluting beads transcatheter arterial chemoembolization(D-TACE) was developed to overcome the drawbacks of C-TACE. D-TACE can load a variety of drugs and ensures sustained and tumor-selective drug delivery while providing permanent embolization.5,6 Unlike lipiodol, drug-eluting beads enable sustained release of chemotherapy drugs in the tumor blood vessels over a longer period of time without elevating systemic concentrations. 8 However, some studies have reported that there is no difference in survival rates between C-TACE and D-TACE, suggesting that combination therapy may be necessary to harness the advantages of D-TACE.9,10
Doxorubicin has been shown to increase the infiltration and aggregation of antigen-specific T cells in the tumor microenvironment (TME) and to modulate the immunosuppressive TME to enhance the potency of PD-L1.11-14 Therefore, combination of doxorubicin and immune checkpoint inhibitors (ICIs) may generate synergistic anti-tumor effects. ICIs are effective in the treatment of intermediate and advanced HCC. 15 Camrelizumab, which was recently approved in China as a second-line treatment for unresectable HCC, shows high receptor occupancy on circulating T lymphocytes, high affinity for PD-1, and different binding epitopes from nivolumab and pembrolizumab.16,17 Additionally, a case report showed that a combination of TACE and camrelizumab for the treatment of unresectable HCC reduced the size of the hepatic lesion and intrahepatic metastatic nodules, and induced necrosis. 18 A recent study demonstrated that D-TACE enhanced immune cell infiltration in tumor tissues, an effect that was not observed in C-TACE-treated tumors. 19 Hence, there is need to determine if D-TACE plus camrelizumab (D-TACE-C) generates a stronger anti-tumor effect than C-TACE plus camrelizumab (C-TACE-C).
In this study, we compared the efficacy and safety of D-TACE-C and C-TACE-C in the treatment of unresectable HCC for the first time using retrospective data
Material and Methods
Study Design and Patient Selection
This was a retrospective study that was approved by the institutional review board of Tongji Medical College, Huazhong University of Science and Technology (UHCT-IEC-SOP-016-03-01). We reviewed the consecutive medical records of unresectable HCC patients who received TACE plus camrelizumab from April 2020 to August 2021. The need of informed consent was waived by the local ethics committee and the institutional review board of the Huazhong University of Science and Technology because clinical data were analyzed retrospectively and anonymously. The reporting of this study conforms to STROBE guidelines, 20 and all patient information is de-identified.
The inclusion criteria for this study were: (1) Adult patients diagnosed with HCC based on the guidelines of the European Association for the Study of the Liver (EASL) and Barcelona Clinic Liver Cancer (BCLC) stage 1 ; (2) Patients classified as B or C in accordance with the BCLC system; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; (4) Patients classified as Child-Pugh class A or B; (5) Patients received the treatment of D-TACE plus camrelizumab or C-TACE plus camrelizumab. The exclusion criteria were: (1) Patients who had received prior immunotherapy or TACE; (2) Patients with incomplete clinical information; (3) Patients who discontinued camrelizumab due to serious adverse events (AEs); (4) Patients classified as Child-Pugh class C or ECOG>1; (5) Patients lost to follow-up.
TACE Procedures
C-TACE Procedures
D-TACE and C-TACE were performed based on our institutional standard protocols, which have been described previously. 21 Briefly, based on the angiography, a catheter or microcatheter was inserted as far as possible into the tumor supplying vessels. After that, the lipiodol (10-20 mL) and epirubicin (10-30 mg) emulsion were injected into the tumor-feeding arteries for embolization with 500-700 μm absorbable gelatin sponge particles (Alicon Medical Co., Hangzhou, China).
D-TACE Procedures
Based on the angiography, a catheter or microcatheter was inserted into the tumor-feeding arteries. CalliSphere beads (Jiangsu Hengrui Medicine Co., Ltd., China) with diameters of 100-300 μm were used as chemoembolization reagent carriers and agents. We dissolved epirubicin to a concentration of 20 mg/mL and then mixed it with the beads using a tee-joint and shook it every 5 minutes for 30 minutes. Finally, the non-ionic contrast agent was added to the solution, and the mixture was subsequently injected into the tumor-feeding arteries at a rate of 1 mL/min until stasis.
Camrelizumab Administration
Patients were treated with camrelizumab within 2-3 weeks after TACE procedures. Camrelizumab was administered intravenously at a dose of 200 mg every 3 weeks. When serious AEs emerged, we reduced the dosage of the drugs or discontinued the therapy and used glucocorticoids or immune-suppressive agents for symptomatic treatment, depending on the severity and the affected organs.
Follow-Up and Evaluation
All Patients included in this study were followed up until 1 August 2021. Patients were evaluated 1 month after the initial treatment, then every 6-9 weeks with laboratory and imaging examinations. Follow-up imaging examinations at 1-3 months were compared with pretreatment imaging to determine disease control rate (DCR) and objective response rate (ORR) according to Modified Response Evaluation Criteria in Solid Tumors (mRECIST). Progression-free survival (PFS), the period between the date of initial TACE and the date of the diagnosis of tumor progression or patient death, was determined by analyzing contrast-enhanced CT or contrast-enhanced magnetic resonance imaging results. The ORR was defined as the percentage of patients with a complete response (CR) or partial response (PR), while DCR was defined as the percentage of patients with CR, PR or stable disease (SD). Adverse events attributed to TACE or camrelizumab, including fever, abdominal pain, nausea, vomiting, reactive cutaneous capillary endothelial proliferation (RCCEP), rash, asthenia, anemia, and hypothyroidism, were observed and recorded.
Statistical Analyses
All analyses were performed using SPSS software, Version 24.0 (IBM, Armonk, New York). Discrete variables were represented by numbers with percentages and were calculated by Chi-square test, and continuous variables were presented as mean ± standard deviation and were calculated by Student’s t-test. Kaplan-Meier method and log-rank test were performed to evaluate the differences in PFS and survival rates between the two groups. The 95% confidence interval (CI) was calculated for median PFS and hazard ratio (HR). Log-rank test was used for univariate analysis, in which variables with P value less than .10 in univariate analysis were added to multivariate analysis. P < .05 indicated a statistically significant difference.
Results
Study Population and Patient Characteristics
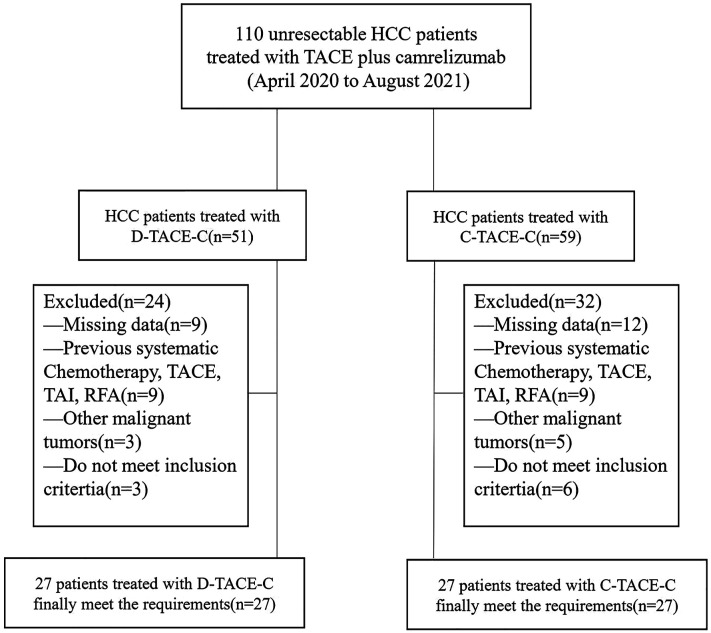
From April 2020 to August 2021, a total of 110 patients who had been diagnosed with HCC at BCLC stage B or C underwent treatment with either D-TACE-C or C-TACE-C. Overall, 56 patients were excluded and 54 patients met the inclusion criteria. Among the patients included, 27 patients received the D-TACE-C treatment, and 27 patients were treated with C-TACE-C (Figure 1). The baseline preoperative characteristics of the 54 patients were listed in Table 1. There was no significant difference in baseline characteristics between the two groups.
Figure 1.
Flowchart of patient selection.
Table 1.
Baseline Characteristics.
| Characteristics | D-TACE-C group(N = 27) (No, %; Mean ± SD) |
C-TACE-C Group (N = 27) (No, %; Mean ± SD) |
P value |
|---|---|---|---|
| Gender | .715 | ||
| Male | 23 (85.2%) | 22 (81.5%) | |
| Female | 4 (14.8%) | 5 (18.5%) | |
| Age (years) | 53.5 ± 10.6 | 52.7 ± 11.8 | .809 |
| ECOG performance | .402 | ||
| 0 | 15 (55.6%) | 18 (66.7%) | |
| 1 | 12 (44.4%) | 9 (33.3%) | |
| BCLC stage | 1 | ||
| B | 9 (33.3%) | 9 (33.3%) | |
| C | 18 (66.7%) | 18 (66.7%) | |
| Hepatitis | .551 | ||
| Hepatitis B | 20 (74.1%) | 18 (66.7%) | |
| Other | 7 (25.9%) | 9(33.3%) | |
| Child-pugh class | .685 | ||
| A | 24 (88.9%) | 23 (85.2%) | |
| B | 3 (11.1%) | 4 (14.8%) | |
| TB (µmol/L) | 16.0 ± 8.0 | 18.3 ± 12.8 | .441 |
| Albumin (g/L) | 37.6 ± 7.4 | 37.0 ± 4.6 | .717 |
| PT(s) | 14.0 ± 1.4 | 13.9 ± 1.1 | .674 |
| AST (µmol/L) | 86.1 ± 101.6 | 56.0 ± 39.7 | .161 |
| ALT (µmol/L) | 54.9 ± 67.3 | 44.4 ± 28.9 | .449 |
| PLR | 155.3 ± 77.0 | 144.0 ± 95.3 | .634 |
| NLR | 3.6 ± 1.9 | 2.8 ± 2.0 | .210 |
| Tumor size (cm) | 9.4 ± 3.6 | 9.4 ± 5.9 | .971 |
| TACE sessions | 4.2 ± 1.8 | 4.0 ± 1.9 | .617 |
| Tumor number | .143 | ||
| ≤3 | 6 (22.2%) | 11 (40.7%) | |
| >3 | 21 (77.8%) | 16 (59.3%) | |
| α-Fetoprotein level | .276 | ||
| ≥400 ng/mL | 12 (44.4%) | 16 (59.3%) | |
| <400 ng/ml | 15 (55.6%) | 11 (40.7%) | |
| Ascites | .552 | ||
| Absent | 25 (92.6%) | 26 (96.3%) | |
| Present | 2 (7.4%) | 1 (3.7%) | |
Note. D-TACE-C: drug-eluting beads transarterial chemoembolization plus camrelizumab; C-TACE-C: conventional transarterial chemoembolization plus camrelizumab; SD: Standard deviation; ECOG: Eastern Cooperative Oncology Group; TB: Total bilirubin; PT: Prothrombin time; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PLR: Platelet-to-lymphocyte ratio; NLR: Neutrophil-to-lymphocyte ratio;
The median follow-up duration was 11.0 months (range, 3-16 months) in the D-TACE-C group and 12.0 months (range, 3-16 months) in the C-TACE-C group. At the end of follow-up, 10 (37.0%) patients in the D-TACE-C group and 11 (40.7%) patients in the C-TACE-C group had died.
Tumor Response
The morphological response of the tumor was verified using abdominal contrast-enhanced CT or MR imaging. In the D-TACE-C group, there was 1 (3.7%) case with CR, 6 (22.2%) cases with PR, 12 (44.4%) cases with SD, and 8 (29.6%) cases with PD. Therefore, the ORR and DCR were 25.9% and 70.4%, respectively. In the C-TACE-C group, there was 1 (3.7%) case with CR, 3 (11.1%) cases with PR, and 7 (25.9%) cases with SD. Therefore, the ORR and DCR of tumor response were 14.8% and 40.7%, respectively. As a result, the chi-square test indicated that DCR in the D-TACE-C group was significantly higher than that in the C-TACE-C group (P=.028), but there was no significant difference in ORR between the two groups (P=.311).
Progression-free Survival and Survival Rates
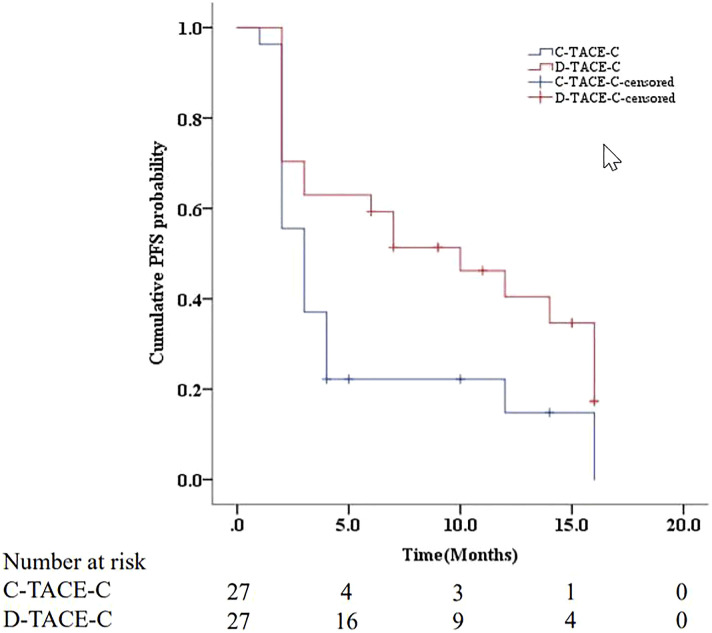
The median PFS was 3 months (95%CI: 2.0, 4.0 months) in the C-TACE-C group, and 10 months (95%CI: 3.7, 16.3 months) in the D-TACE-C group. The median PFS between the two groups was significantly different (P=.017) (Figure 2). Univariate analysis indicated that none of the baseline preoperative characteristics were associated with PFS (Table 2). However, treatment with D-TACE-C was associated with better PFS (P=.042) in patients with unresectable HCC. Median survival time was not attained, and the 6-month and 12-month survival rates in D-TACE-C group and C-TACE-C group were 85.2% versus 79.4% (P=.646) and 65.2% versus 65.1% (P=.903), respectively.
Figure 2.
Kaplan-Meier curve of cumulative PFS in HCC patients treated with C-TACE-C or D-TACE-C.
Table 2.
Univariate analysis of prognostic factors for progression-free survival.
| Variables | HR (95% CI) | P value |
|---|---|---|
| Gender | ||
| Female | Reference | |
| Male | .971(.448,2.107) | .942 |
| ECOG performance | ||
| 1 | Reference | |
| 0 | 1.407(.735,2.696) | .303 |
| Hepatitis | ||
| Other | Reference | |
| Hepatitis B | .610(.314,1.185) | .145 |
| Child-pugh class | ||
| B | Reference | |
| A | .519(.227,1.189) | .121 |
| Age (years) | .995(.967,1.024) | .724 |
| AST (µmol/L) | .999 (.995,1.003) | .590 |
| ALT (µmol/L) | 1.000 (.995, 1.005) | .919 |
| PLR | 1.002(.998, 1.005) | .356 |
| NLR | 1.082(.931, 1.257) | .306 |
| Albumin (g/L) | .996(.949, 1.046) | .880 |
| TB (µmol/L) | 1.006(.978, 1.036) | .660 |
| PT (s) | 1.006(.763, 1.326) | .967 |
| Tumor size | 1.007(.940, 1.079) | .840 |
| TACE sessions | .942(.828, 1.072) | .365 |
| BCLC stage | ||
| C | Reference | |
| B | .628(.321, 1.226) | .173 |
| Tumor number | ||
| >3 | Reference | |
| ≤3 | 1.0.87(.569, 2.077) | .800 |
| α-Fetoprotein level | ||
| ≥400 ng/mL | Reference | |
| <400 ng/mL | 1.172 (.633, 2.168) | .613 |
| Ascites | ||
| Present | Reference | |
| Absent | 1.917 (.459, 8.004) | .372 |
| Treatment method | ||
| C-TACE-C | Reference | |
| D-TACE-C | .516(.273,0.975) | .042 |
Note. HR: Hazard ratio; CI: Confidence interval; ECOG: Eastern Cooperative Oncology Group; TB: Total bilirubin; PT: Prothrombin time; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
Adverse Events
Most of the AEs associated with TACE occurred within two weeks after TACE. In the D-TACE-C group, AEs related to TACE occurred in 23 patients (85.2%), including 14 patients (51.9%) with fever, 11 patients (40.7%) with nausea, and 8 patients (29.6%) with vomiting. In the C-TACE-C group, 24 patients (88.9%) developed fever (n=12), abdominal pain (n=9), nausea, and vomiting (n=9). The Chi-square test indicated that there was no significant difference between the two groups. In addition, the TACE-induced AEs were alleviated or eliminated after symptomatic treatment.
AEs related to camrelizumab are shown in Table 3. During the follow-up period, a total of 48 (88.9%) patients developed at least one type of AE after treatment with camrelizumab, and there was no significant difference in all kinds of AEs between the two groups. Moreover, no patients developed severe AEs (more than grade 3) and no treatment-related deaths occurred in this study.
Table 3.
Adverse Events Related to Camrelizumab in Two Groups.
| Adverse event | D-TACE-C (n = 27) | C-TACE-C (n = 27) | P-value |
|---|---|---|---|
| RCCEP | 23 (85.2%) | 18 (66.7%) | .111 |
| Rash | 6 (22.2%) | 8 (29.6%) | .535 |
| Asthenia | 6 (22.2%) | 4 (14.8%) | .484 |
| Anemia | 3 (11.1%) | 2 (7.4%) | .639 |
| Hypothyroidism | 1 (3.7%) | 2 (7.4%) | .552 |
Note. RCCEP: Reactive cutaneous capillary endothelial proliferation.
Discussion
D-TACE is a novel drug delivery embolization method designed to deliver a higher and more sustained release of drug into the tumor vessels and a low release of the drug into systemic circulation. This maximizes the effectiveness of the drug and significantly reduces its systemic toxicity.22,23 Despite the superior design concept of D-TACE, a previous study demonstrated that D-TACE enhanced tumor response but did not improve survival in patients with BCLC stage A and B. 24 Since the mode of drug-delivery between D-TACE and C-TACE is different, there is need to explore the difference in efficacy between a combination of D-TACE and ICIs, (the drugs recommended for advanced HCC 25 ), and a combination of C-TACE and ICIs.
We found that the median PFS in patients with unresectable HCC who had undergone D-TACE-C was higher than for patients who underwent C-TACE-C. A retrospective study showed that the median PFS in patients with unresectable HCC who received a combination of D-TACE and Apatinib (D-TACE-A) was 7 months, which was lower than the median PFS in the present study. 7 Moreover, the study demonstrated that D-TACE-A and C-TACE plus Apatinib (C-TACE-A) had similar efficacy. These findings suggested that a combination of ICIs and TACE (especially D-TACE) was more beneficial for patients with unresectable HCC than a combination of anti-angiogenic therapy and TACE. Recently, JSH Consensus Statements and Recommendations indicated that D-TACE may be more advantageous for patients with bilobular multiple HCCs and larger HCCs, and that C-TACE is theoretically more effective than D-TACE for small HCCs. 26 In addition, a subgroup analysis in a multicenter RCT showed that D-TACE was associated with significantly higher response rates in patients with bilobular multifocal disease. 22 In the present study, tumor size in two groups was at least 9 cm and tumor numbers were more than 3. For these reasons, it is likely that D-TACE may bring better PFS than C-TACE for patients.
We also found that treatment with D-TACE-C led to better tumor response and higher DCR (70.4% vs. 40.7%, P=.028) than treatment with C-TACE-C. Recently, a study reported that TAE-treated (non-loaded beads) tumors had more surviving tumor cells under stress after 3 days, irrespective of bead size, compared with DEB-TACE-treated (doxorubicin-eluting beads) tumors. 27 Similarly, the unsustained concentration or uneven distribution of doxorubicin allowed more tumors cells to survive leading to tumor progression. Moreover, due to the multiple blood vessels supplying the tumor and the fluidity of lipiodol, lipiodol is likely to be washed out, resulting in poor deposition, tumor vascular recanalization, tumor recurrence, and metastasis. 28 In contrast, drug-eluting beads ensure the continuous and stable concentration of doxorubicin in the tumor, and the microspheres can permanently embolize the target tumor-feeding arteries.29,30
Univariate COX regression analysis showed that treatment was the only factor associated with PFS, with patients who had received D-TACE-C having better PFS. The other baseline preoperative characteristics were not associated with PFS. This result verified our hypotheses that camrelizumab may improve the efficacy of TACE in patients with unresectable HCC.
D-TACE has been shown to increase the infiltration of immune cells in tumor tissues, while C-TACE reduces the infiltration of immune cells. 19 Therefore, the addition of ICI is expected to enhance the therapeutic effect of D-TACE, and may eventually prolong the OS of patients. However, the results showed that there was no difference in 6 and 12-month survival rates between the two groups. This may be due to the small sample size and short follow-up time. Since tumor burden of the patients was large, multiple TACE sessions were required to allow more immune cells to infiltrate the whole tumor tissue, and eventually prolong the OS.
Although some studies reported that the incidences of TACE-induced AEs were higher in the C-TACE group than in the D-TACE group,22,31 some meta-analysis showed no significant difference in AEs between C-TACE and D-TACE.32,33 These findings are consistent with findings from this study. Similar to other studies,16,34,35 RCCEP, rash, and asthenia were the most common AEs associated with camrelizumab. In addition, there is no difference in the camrelizumab-associated AEs between the two groups. Overall, all AEs were clinically controllable and self-limiting, and were alleviated or eliminated after symptomatic treatment.
There were some limitations in this study. First, this was a retrospective study, with the potential risk of selection bias. Second, since the data in this study came from a single-center with a small sample size, and a multicenter prospective randomized trial is needed. Lastly, due to the relatively short follow-up time, we did not get the median OS and could not determine if D-TACE-C induced better OS than C-TACE-C.
Conclusions
This is the first study comparing the efficacy and safety of D-TACE plus camrelizumab with that of C-TACE plus camrelizumab. In conclusion, the treatment of D-TACE-C is safe and well-tolerated, and may bring better PFS and tumor response than C-TACE-C in patients with unresectable HCC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grant from National Nature Science Foundation of China (No.81601580 and No.81873919), Fundamental Research Funds for the Central Universities (2021yjsCXCY101)
References
- 1.EASL Clinical Practice Guidelines . Management of hepatocellular carcinoma. Journal of hepatology. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238-iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 4.Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World Journal of Gastroenterology. 2018;24(2):161-169. doi: 10.3748/wjg.v24.i2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouri YM, Kim JH, Yoon HK, Ko HK, Shin JH, Gwon DI. Update on Transarterial Chemoembolization with Drug-Eluting Microspheres for Hepatocellular Carcinoma. Korean journal of radiolog . 2019;20(1):34-49. doi: 10.3348/kjr.2018.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. Journal of hepatology. Mar. 2007;46(3):474-481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Chen L, Cao Y, et al. Efficacy of drug-eluting beads transarterial chemoembolization plus apatinib compared with conventional transarterial chemoembolization plus apatinib in the treatment of unresectable hepatocellular carcinoma. Cancer Management and Research. 2021;13:5391-5402. doi: 10.2147/cmar.S314762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sottani C, Poggi G, Quaretti P, et al. Serum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheres. Anticancer research. 2012;32(5):1769-1774. [PubMed] [Google Scholar]
- 9.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255-264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie ZZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45(2):190-200. doi: 10.1111/hepr.12450. [DOI] [PubMed] [Google Scholar]
- 11.Chao Y, Liang C, Tao H, et al. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Science advances. Sci Adv. 2020;6(10):eaaz4204. doi: 10.1126/sciadv.aaz4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater (Deerfield Beach, Fla). 2019;31(34):e1803322. doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 13.Nam J, Son S, Park KS, Zou W, Shea LD, Moon JJ. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4(6):398-414. doi: 10.1038/s41578-019-0108-1. [DOI] [Google Scholar]
- 14.Zhang Z, Yu X, Wang Z, Wu P, Huang J. Anthracyclines potentiate anti-tumor immunity: a new opportunity for chemoimmunotherapy. Cancer Letters. 2015;369(2):331-335. doi: 10.1016/j.canlet.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 15.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London, England). 2017;389(10088):2492-2502. doi: 10.1016/s0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571-580. doi: 10.1016/s1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 17.Markham A, Keam SJ. Camrelizumab: first global approval. Drug . 2019;79(12):1355-1361. doi: 10.1007/s40265-019-01167-0. [DOI] [PubMed] [Google Scholar]
- 18.Xin H, Zhang C, Ding Z, Zhang M, Ding G, Li N. TACE plus PD-1 inhibitor (Camrelizumab) treatment for bridging to tumor resection in HCC: case reports. Clinics and research in hepatology and gastroenterology. 2021; 46(1):101777. doi: 10.1016/j.clinre.2021.101777. [DOI] [PubMed] [Google Scholar]
- 19.Doemel LA, Santana JG, Savic LJ, et al. Comparison of metabolic and immunologic responses to transarterial chemoembolization with different chemoembolic regimens in a rabbit VX2 liver tumor model. Eur Radiol 2021;32:2437-2447. doi: 10.1007/s00330-021-08337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Ren Y, Ge S, et al. Transarterial chemoembolization in treatment-naïve and recurrent hepatocellular carcinoma: a propensity-matched outcome and risk signature analysis. Frontiers in oncology. 2021;11:662408. doi: 10.3389/fonc.2021.662408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41-52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(8):2563-2567. doi: 10.1158/1078-0432.Ccr-05-2225. [DOI] [PubMed] [Google Scholar]
- 24.Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. Journal of hepatology. 2012;57(6):1244-1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Greten TF, Abou-Alfa GK, Cheng AL, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J Immunother Cancer. 2021;9(9):e002794. doi: 10.1136/jitc-2021-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver cancer. 2021;10(3):181-223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes AC, Nishiofuku H, Polak U, et al. Effect of bead size and doxorubicin loading on tumor cellular injury after transarterial embolization and chemoembolization in a rat model of hepatocellular carcinoma. Nanomedicine : nanotechnology, biology, and medicine. 2021;24:102465. doi: 10.1016/j.nano.2021.102465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CD, Qi YG, Peng SY. Lipiodolization with or without gelatin sponge in hepatic arterial chemoembolization for hepatocellular carcinoma. Chinese medical journal . 1994;107(3):209-215. [PubMed] [Google Scholar]
- 29.Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Intervent Radiol : JVIR. Aug. 2006;17(8):1335-1343. doi: 10.1097/01.Rvi.0000228416.21560.7f. [DOI] [PubMed] [Google Scholar]
- 30.Lewis AL. DC Bead: a major development in the toolbox for the interventional oncologist. Expet Rev Med Dev. 2009;6(4):389-400. doi: 10.1586/erd.09.20. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda M, Inaba Y, Tanaka T, et al. A prospective randomized controlled trial of selective transarterial chemoembolization using drug-eluting beads loaded with epirubicin versus selective conventional transarterial chemoembolization using epirubicin-lipiodol for hepatocellular carcinoma. Am.J.Clin.Oncol. 2020;38(15):4518. doi: 10.1200/JCO.2020.38.15_suppl.4518. [DOI] [Google Scholar]
- 32.Liang B, Makamure J, Shu S, Zhang L, Sun T, Zheng C. Treatment response, survival, and safety of transarterial chemoembolization with calliSpheres(®) microspheres versus conventional transarterial chemoembolization in hepatocellular carcinoma: a meta-analysis. Frontiers in oncology. 2021;11:576232. doi: 10.3389/fonc.2021.576232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48(6):571-577. doi: 10.1016/j.dld.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832-842. doi: 10.1016/s1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162-1174. doi: 10.1016/s1470-2045(21)00302-8. [DOI] [PubMed] [Google Scholar]