Abstract
Background
Lyme borreliosis is the most prevalent arthropod-borne infection in the Northern Hemisphere. In Europe, Borrelia afzelii is predominantly involved in cutaneous manifestations, Borrelia garinii and Borrelia bavariensis in neurological manifestations, and Borrelia burgdorferi sensu stricto in articular ones. Liver impairement is not classical in Lyme borreliosis. Diagnosis is currently mainly based on serological testing, and is challenging in immunocompromised patients.
Case presentation
We report the first case of B. garinii infection revealed by liver involvement in an immunocompromised man. A 73-year-old man with marginal zone lymphoma, treated with bendamustine and rituximab, developed intermittent fever and inflammatory syndrome. Microbial investigations were all negative and FDG-PET showed complete remission of the lymphoma. Three months later, liver biopsy was performed and histology revealed spirochetes-like bacteria. Microbial diagnosis was performed by 16S rDNA sequencing, flagellin (flaB) gene sequencing and multi-locus sequence typing and identified B. garinii. The patient recovered successfully after a three weeks course of antibiotics. Diagnosis was challenging because Borrelia hepatic involvement is unusual and no erythema migrans nor tick bite were notified.
Conclusion
This case highlights that unexplained fever and inflammatory syndrome in immunocompromised patients warrants specific investigations to identify bacteria such as spirochetes.
Keywords: Borrelia garinii, 16S rDNA, Liver involvement, Kupffer cell hyperplasia, Warthin Starry stain
Background
Lyme borreliosis is caused by the tick-borne spirochetes of the Borrelia burgdorferi sensu lato (s.l.) complex and is the most prevalent arthropod-borne infection in the Northern Hemisphere. Its incidence increased over the last few decades in many European countries, including France [1, 2]. This multisystem disease is caused by infection with spirochaetal bacteria of the B. burgdorferi s.l. complex. These bacteria are transmitted to humans and other vertebrate hosts via the bite of infected Ixodes spp. ticks, mainly Ixodes ricinus in Europe [3].
Five Borrelia species are mainly pathogenic to humans: Borrelia afzelii, Borrelia burgdorferi sensu stricto (s.s.), Borrelia garinii, Borrelia bavariensis and, less often reported, Borrelia spielmanii [3]. All five species are present in Europe, although B. afzelii and, to a lesser extent, B. garinii predominate, whereas B. burgdorferi s.s. is the main species responsible for Lyme borreliosis in North America [3].
Borrelia infection in humans can cause a range of clinical features. Patients may present with a variety of symptoms, which can vary according to the stage of the disease and the level of bacterial dissemination through the blood and tissues [3]. Localized infection is typically manifested by erythema migrans skin lesion. It appears in the early stages of the disease and presents at the initial site of inoculation. Early disseminated disease is characterized by neuroborreliosis (mainly presents as meningoradiculitis and cranial nerve palsy), Lyme arthritis, or more rarely, multiple erythema migrans, borrelial lymphocytoma or Lyme carditis. Late Lyme borreliosis usually manifests as chronic Lyme arthritis, acrodermatitis chronica atrophicans, and late neurological manifestations [4]. Of the various objective clinical presentations, erythema migrans is the most common.
Infection with certain Borrelia spp. has been associated with specific disseminated clinical manifestations. Current knowledge is that B. afzelii is predominantly involved in cutaneous manifestations in Europe, is often found in the localized incipient form of the infection and in acrodermatitis chronica atrophicans, B. garinii and B. bavariensis in neurological manifestations, and B. burgdorferi s.s. in articular ones [3].
Liver impairement is not classical in Lyme borreliosis. We here report the first case of liver involvement associated with B. garinii infection.
Case presentation
In January 2020, a 73-year-old French man with no medical history, was diagnosed with a marginal zone lymphoma. He received chemotherapy consisting of bendamustine and rituximab. After the fourth cycle of chemotherapy, he developed inflammation with elevated serum C-reactive protein (150 mg/L) without any clinical symptoms. No abnormal findings were observed on physical examination. Fluorodeoxyglucose (FDG) positron emission tomography (PET) demonstrated lymphoma complete remission. He nevertheless received two additional cycles of chemotherapy until May 2020. Then, he developed intermittent fever and nocturnal sweat. He was admitted to hospital in July 2020. Except a temperature of 38.7 °C, physical examination was normal. No tick bite or erythema migrans was notified. Laboratory tests revealed the following: alkaline phosphatase 360 U/L, ALAT 64 U/L, ASAT 67 U/L, white blood cell count 8.4 g/L. His immunity state was obviously compromised with deep lymphopenia (0.54 g/L) and hypogammaglobulinemia (2.6 g/L). Computerized tomography-scan as well as cardiac echography were normal. Serological tests for B. burgdorferi s.l., Francisella spp., Bartonella spp., Brucella spp., Rickettsia spp. and Coxiella spp. were negative. Blood and urine culture, PCR targeting Tropheryma whipplei in blood as well as tests for anti-nuclear antibodies and rheumatoid factor, were all negative. Bone marrow biopsy and a new FDG-PET confirmed that lymphoma was still in complete remission. As patient’s state was worsening, we started prednisone at the dose of 0.5 mg/kg with dramatic but only transient improvement. During the three months follow-up, the patient presented slightly elevated levels of cholestasis parameters that were initially attributed to chronic inflammation. As fever persisted, liver biopsy was performed. Histology showed significant sinusoidal dilation (peliosis) and a rich inflammatory infiltrate composed predominantly of neutrophils. The latter were intra-sinusoidal and infiltrated the portal spaces and bile ducts (neutrophilic cholangitis/cholangiolitis). Additional findings included Kupffer cell hyperplasia with hemophagocytosis and extramedullary hematopoiesis. Warthin Starry stain highlighted spirochetes-like bacteria within hepatic sinusoids (Fig. 1).
Fig. 1.
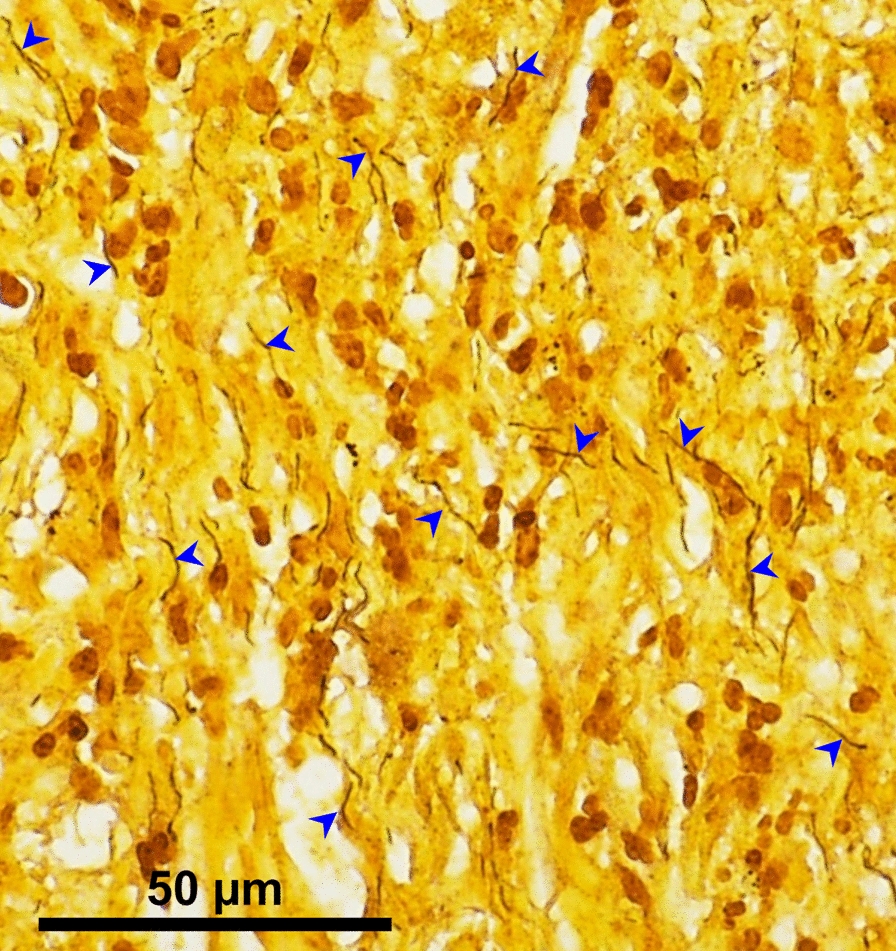
Images of human liver biopsy specimens. Warthin Starry silver nitrate staining showing spirochete-like bacteria (blue arrowheads) (original magnification × 400)
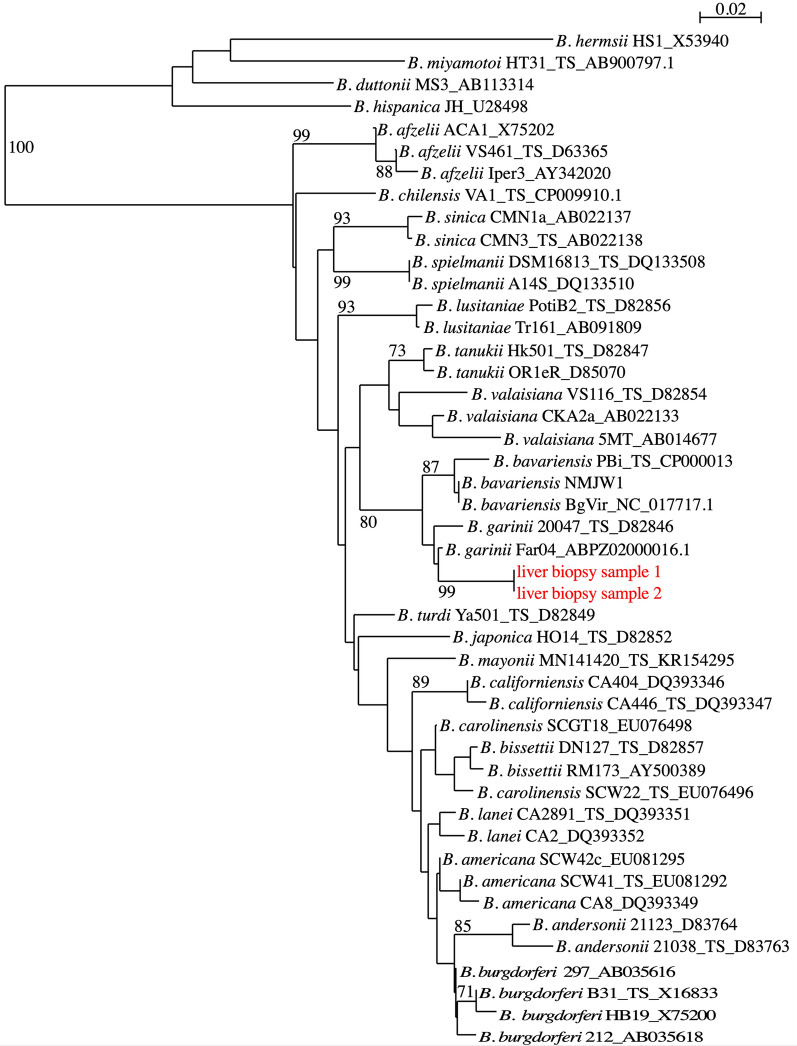
Standard bacterial cultures of liver biopsy were negative. A broad-range 16S rRNA gene-based PCR performed on a DNA extract from liver biopsy led to a 1457 bp sequence (GenBank accession number: MZ227021) that showed 100% similarities with the sequence of B. garinii strain 20047 (GenBank accession number CP028861.1). To confirm the species B. garinii, DNA extract of liver biopsy was sent to the French National Reference Center (NRC) for Borrelia (Strasbourg, France). Liver biopsy was also sent to the NRC for borrelial culture and PCR. Specific PCR for B. burgdoferi s.l. targeting the flaB gene turned out strongly positive (Cycle threshold value 30). Identification at the species level was confirmed by the phylogenetic analysis of the flaB gene, which showed that the isolate was clustered within B. garinii species (Fig. 2).
Fig. 2.
Phylogenetic tree of Borrelia taxa based on flagellin gene (158 nt). DNA extracts from two different areas of the liver biopsy were analyzed (indicated in red). The phylogenetic analyses were generated with the BioNeighbor-Joining method. The phylogeny presented is based on the alignment of 158-bp sequences of flaB gene from 44 reference sequences representing the current known diversity in the genus Borrelia and the sequence amplified in the case report presented herein. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The tree is not rooted and drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are represented in the units of the number of base substitutions per site. Evolutionary analyses were conducted using SeaView. All sequences are labeled by species, strain name, and GenBank accession number. TS type strain
Multilocus sequence typing (MLST) was performed on eight housekeeping genes [5]. Complete allelic profile was obtained for five genes: clpA#43, nifS#30, pepX#90, pyrG#87, recG#36 (www.pubmlst.org/borrelia). We obtained a new allele for the clpX gene, closest to clpX#28 (one nucleotide difference). All of these sequences were confirmed to originate from the species B. garinii by comparison to sequences in the MLST database.
After establishing this diagnosis, we confirmed that patient was truly seronegative two months after the onset of symptoms, with two different first-step serological tests (Liaison® Borrelia IgG and IgM, Diasorin and Enzygnost IgG and IgM ELISA, Siemens).
The patient was treated with ceftriaxone (2 g/day) for three weeks. The patient recovered successfully, and no signs of recurrence was observed in the following six months.
Discussion
In humans, only five cases of clinical liver damage have been reported to date in Lyme borreliosis [6–10]. Chavanet et al. reported a case of febrile granuloma hepatitis in a 46-year-old man with no medical history [6]. Goellner et al. described a case of hepatitis in a 73-year-old woman that appeared to be the result of direct tissues invasion by the spirochete [8]. Histopathology of liver biopsy showed ballooning of hepatocytes, marked hepatocyte mitotic activity, prominent microvesicular fat, Kupffer cell hyperplasia, and sinusoidal infiltration by mononuclear cells and polynuclear neutrophils. Dieterle silver-staining showed spirochetes within hepatic sinusoids and parenchyma, but molecular identification was not performed and culture remained negative. Dadamessi et al. described a case of liver injury in a 71-year-old man who presented with febrile jaundice; liver biopsy revealing a non-specific sinusoidal inflammatory infiltrate without necrosis [7]. Zanchi et al. reported a case of Lyme borreliosis with necrotizing granulomas and eosinophilic infiltration of the hepatic parenchyma [9]. Then, Middelveen et al. described a case of granulomatous hepatitis associated with chronic Lyme borreliosis in a 53-year-old woman [10]. Spirochetes were observed within parenchyma of the liver biopsy tissue on histological examination, and identification of B. burgdoferi s.s was performed from positive blood cultures using molecular methods [10]. Although it is possible to see spirochetes on liver biopsy in patients with Lyme borreliosis and hepatitis, in the majority of these cases, no organisms are identified and diagnosis relies on positive serological tests. Spirochetes cannot be detected by Gram staining, whereas Warthin Starry staining revealed them in liver biopsy in the present case, allowing the subsequent diagnosis of B. garinii infection by 16S rRNA gene-based molecular methods. This is the third case where the spirochete was directly demonstrated in liver biopsy.
The pathogenesis of liver injury in patients with Lyme borreliosis includes an interplay of direct hepatic invasion by the spirochete and immunologic responses. Lyme borreliosis can result in a variety of histologic abnormalities in the liver, in particular, sinusoidal infiltration by a mixed inflammatory infiltrate [8]. Rarely, B. burgdoferi s.l. can result in granulomatous hepatitis.
B. burgdoferi s.l. strains are not known to produce toxins. Most tissue damage seems to result from host inflammatory reactions. The intensity of inflammatory response varies according to the Borrelia species that causes an infection [11].
In the present case, the patient did not remember a tick bite or erythema migrans, as also described in other cases [6, 7, 9]. Erythema migrans is the most common skin finding seen in more than 80% of patients with early Lyme borreliosis [12]. It typically develops 7 to 14 days (range, 3 to 30) after tick detachment [13]. The erythema migrans lesions are often found in or near the armpit, inguinal region, or popliteal fossa but can occur in any part of the body.
Gastrointestinal signs and symptoms are common in the early stages of the disease. In a study of 314 patients, approximately 10% of the patients had symptoms that were suggestive of hepatitis [14]. In another study, 27% of the patients had subclinical hepatitis during the early stages of disease [15]. Patients with early disseminated Lyme borreliosis are more likely to have abnormal liver function test finding than are patients with localized disease [16]. However, elevations in aspartate aminotransferase may indicate Lyme disease-associated myositis in some patients and may not be related to underlying hepatic injury.
In our patient, serological assays that detect antibodies for B. burgdorferi s.l. were negative while it is generally accepted that patients with disseminated Lyme borreliosis with symptoms for more than six weeks usually reveal positive Borrelia serology [17]. This was explained by the lymphopenia and the reduction of total IgA/IgM/IgG concentrations, in agreement with other observations [18]. Cultivation and isolation from clinical material is a golden standard for confirmation of Borrelia infection [19]. However, many factors influence in vitro Borrelia growth such as medium ingredients and its pH, temperature of incubation, the presence of contaminants, sample’s cell density, number of Borrelia strains in the sample, antecedent antibiotic therapy or capacity of particular Borrelia species to grow [17, 19, 20]. Indeed, Borrelia culture is impractical for routine clinical use [17, 19, 20]. In the present case, diagnosis was established using molecular methods, directly performed on liver biopsy, and permitted the official identification of the bacterium. The gold standard for genotyping of B. burgdorferi s.l. nowadays is MLST, which undergo slow evolution and show nearly neutral variation [5]. Of interest, MLST analysis of B. garinii isolates from bird-derived ticks, questing ticks and humans revealed that there was little overlap among genotypes from different continents, no geographical structuring within Europe, and no evident association pattern detectable among B. garinii genotypes from ticks feeding on birds, questing ticks or human isolates [21]. This provides supporting evidence that birds act as important reservoirs for B. garinii and are a main source of infection of this species to ticks and ultimately humans (through the bite of an infected tick).
Conclusion
This case illustrates the potential for seronegative Lyme borreliosis in patients with immunomodulatory therapy, and the need for further investigations to avoid missing or delaying the accurate diagnosis. Diagnosis can be difficult because erythema migrans is not always present, and Borrelia liver involvement rarely occurs.
Acknowledgements
We are grateful to Pr Benoit Jaulhac (CHRU Strasbourg) for his expertise on Borrelia and to Lamia Azzi-Martin (Univ. Bordeaux, UFR Sciences médicales) for technical picture assistance.
Abbreviations
- FDG-PET
Fluorodeoxyglucose positron emission tomography
- MLST
Multilocus sequence typing
Authors’ contributions
PD and VV clinically managed the patient; SK, ETR, AM and OP contributed to the microbiological diagnosis; PD, SK, ETR, AM and OP wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. According to the French legislation, Ethical approval for a single case is not required, as long as the data kept anonymous, and the investigations do not imply human genetic results.
Consent for publication
Written informed consent to publish this paper was obtained from the patient.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Septfons A, Figoni J, Gautier A, Soullier N, de Valk H, Desenclos JC. Increased awareness and knowledge of Lyme Borreliosis and tick bite prevention among the general population in France: 2016 and 2019 health barometer survey. BMC Public Health. 2021;21(1):1808. doi: 10.1186/s12889-021-11850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. ClinMicrobiol Infect. 2011;17(1):69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 3.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 4.Figoni J, Chirouze C, Hansmann Y, Lemogne C, Hentgen V, Saunier A, et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): prevention, epidemiology, diagnosis. Med Mal Infect. 2019;49(5):318–334. doi: 10.1016/j.medmal.2019.04.381. [DOI] [PubMed] [Google Scholar]
- 5.Margos G, Notter I, Fingerle V. Species identification and phylogenetic analysis of Borrelia burgdorferi sensu lato using molecular biological methods. Methods Mol Biol. 2018;1690:13–33. doi: 10.1007/978-1-4939-7383-5_2. [DOI] [PubMed] [Google Scholar]
- 6.Chavanet P, Pillon D, Lancon JP, Waldner-Combernoux A, Maringe E, Portier H. Granulomatous hepatitis associated with Lyme disease. Lancet. 1987;2(8559):623–624. doi: 10.1016/S0140-6736(87)93009-1. [DOI] [PubMed] [Google Scholar]
- 7.Dadamessi I, Brazier F, Smail A, Delcenserie R, Dupas JL, Capron JP. Hepatic disorders related to Lyme disease Study of two cases and a review of the literature. Gastroenterol Clin Biol. 2001;25(2):193–196. [PubMed] [Google Scholar]
- 8.Goellner MH, Agger WA, Burgess JH, Duray PH. Hepatitis due to recurrent Lyme disease. Ann Intern Med. 1988;108(5):707–708. doi: 10.7326/0003-4819-108-5-707. [DOI] [PubMed] [Google Scholar]
- 9.Zanchi AC, Gingold AR, Theise ND, Min AD. Necrotizing granulomatous hepatitis as an unusual manifestation of Lyme disease. Dig Dis Sci. 2007;52(10):2629–2632. doi: 10.1007/s10620-006-9405-9. [DOI] [PubMed] [Google Scholar]
- 10.Middelveen MJ, McClain SA, Bandoski C, Israel JR, Burke J, MacDonald AB, et al. Granulomatous hepatitis associated with chronic Borrelia burgdorferi infection: a case report. Research. 2014;1:875. doi: 10.13070/rs.en.1.875. [DOI] [Google Scholar]
- 11.Strle K, Drouin EE, Shen S, El Khoury J, McHugh G, Ruzic-Sabljic E, et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis. 2009;200(12):1936–1943. doi: 10.1086/648091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steere AC. Lyme disease. N Engl J Med. 2001;345(2):115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 13.Wormser GP. Clinical practice. Early Lyme disease. N Engl J Med. 2006;354(26):2794–2801. doi: 10.1056/NEJMcp061181. [DOI] [PubMed] [Google Scholar]
- 14.Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, et al. The early clinical manifestations of Lyme disease. AnnInternMed. 1983;99(1):76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 15.Kazakoff MA, Sinusas K, Macchia C. Liver function test abnormalities in early Lyme disease. Arch FamMed. 1993;2(4):409–413. doi: 10.1001/archfami.2.4.409. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz HW, Dworkin B, Forseter G, Nadelman RB, Connolly C, Luciano BB, et al. Liver function in early Lyme disease. Hepatology. 1996;23(6):1412–1417. doi: 10.1002/hep.510230617. [DOI] [PubMed] [Google Scholar]
- 17.Jaulhac B, Saunier A, Caumes E, Bouiller K, Gehanno JF, Rabaud C, et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med Mal Infect. 2019;49(5):335–346. doi: 10.1016/j.medmal.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Wagemakers A, Visser MC, de Wever B, Hovius JW, van de Donk N, Hendriks EJ, et al. Case report: persistently seronegative neuroborreliosis in an immunocompromised patient. BMC Infect Dis. 2018;18(1):362. doi: 10.1186/s12879-018-3273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branda JA, Steere AC. Laboratory diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2021 doi: 10.1128/CMR.00018-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohr B, Fingerle V, Norris DE, Hunfeld KP. Laboratory diagnosis of Lyme borreliosis: current state of the art and future perspectives. CritRev Clin Lab Sci. 2018;55(4):219–245. doi: 10.1080/10408363.2018.1450353. [DOI] [PubMed] [Google Scholar]
- 21.Norte AC, Margos G, Becker NS, Albino Ramos J, Nuncio MS, Fingerle V, et al. Host dispersal shapes the population structure of a tick-borne bacterial pathogen. Mol Ecol. 2020;29(3):485–501. doi: 10.1111/mec.15336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.