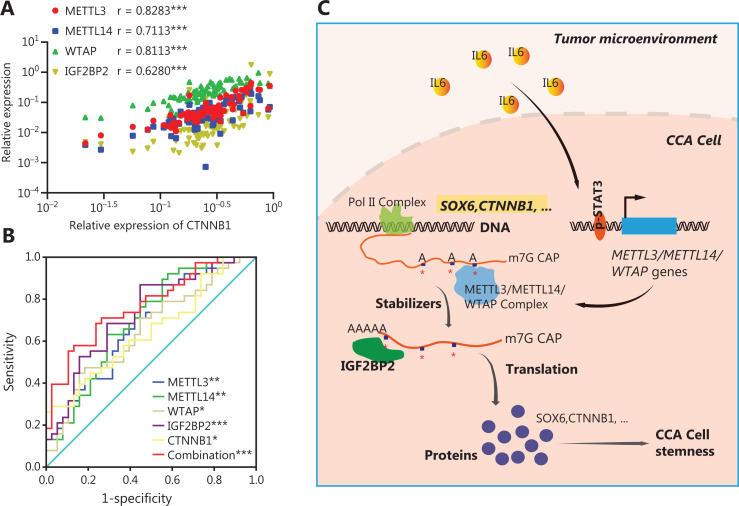
Figure 7.
Clinical relevance of m6A writers in CCA and schematic representations of pathways linking m6A, IL-6, and CCA cell stemness. (A) CTNNB1 expression positively correlated with both m6A writers and IGF2BP2 in 38 paired CCA tumor and adjacent normal tissues. Spearman’s correction was used to analyze the significance (***P < 0.001). (B) Diagnostic value of m6A writers and IGF2BP2, and CTNNB1 for CCA. The areas under the curve were 0.6794 for METTL3 [95% confidence interval (CI): 0.5597–0.7990, P < 0.01], 0.6994 for METTL14 (95% CI: 0.5816–0.8172; P < 0.01), 0.6524 for WTAP (95% CI: 0.5291–0.7756; P < 0.05), 0.7313 for IGF2BP2 (95% CI: 0.6183–0.8443; P < 0.001), 0.6496 for CTNNB1 (95% CI: 0.5264–0.7728; P < 0.05), and 0.7722 for the combination (95% CI: 0.6679–0.8764; P < 0.001). The sensitivity and specificity at each cutoff point were as follows: 86.84% and 44.74% for METTL3, 92.11% and 42.11% for METTL14, 47.37% and 81.58 % for WTAP, 86.84% and 55.26 % for IGF2BP2, 28.95% and 97.37% for CTNNB1, and 68.42 % and 76.32% for the combination. (C) A working model for the function of m6A writers in facilitating cancer cell stemness in CCA. In the tumor microenvironment, the inflammatory factor, IL-6, can trigger the STAT3 pathway. The activated STAT3 located at the METTL3, METTL14, and WTAP gene loci then increased their expressions. This increases the expression of m6A genes, which contributes to an increase in m6A methylation of stemness-related transcripts, including SOX6 and CTNNB1. Methylated stemness-related transcripts are subsequently recognized by IGF2BP2, to maintain their mRNA stability and expression, resulting in abnormal CCA cell stemness and pathogenesis of CCA.