Fig. 3 |. Mitochondrial fusion and fission.
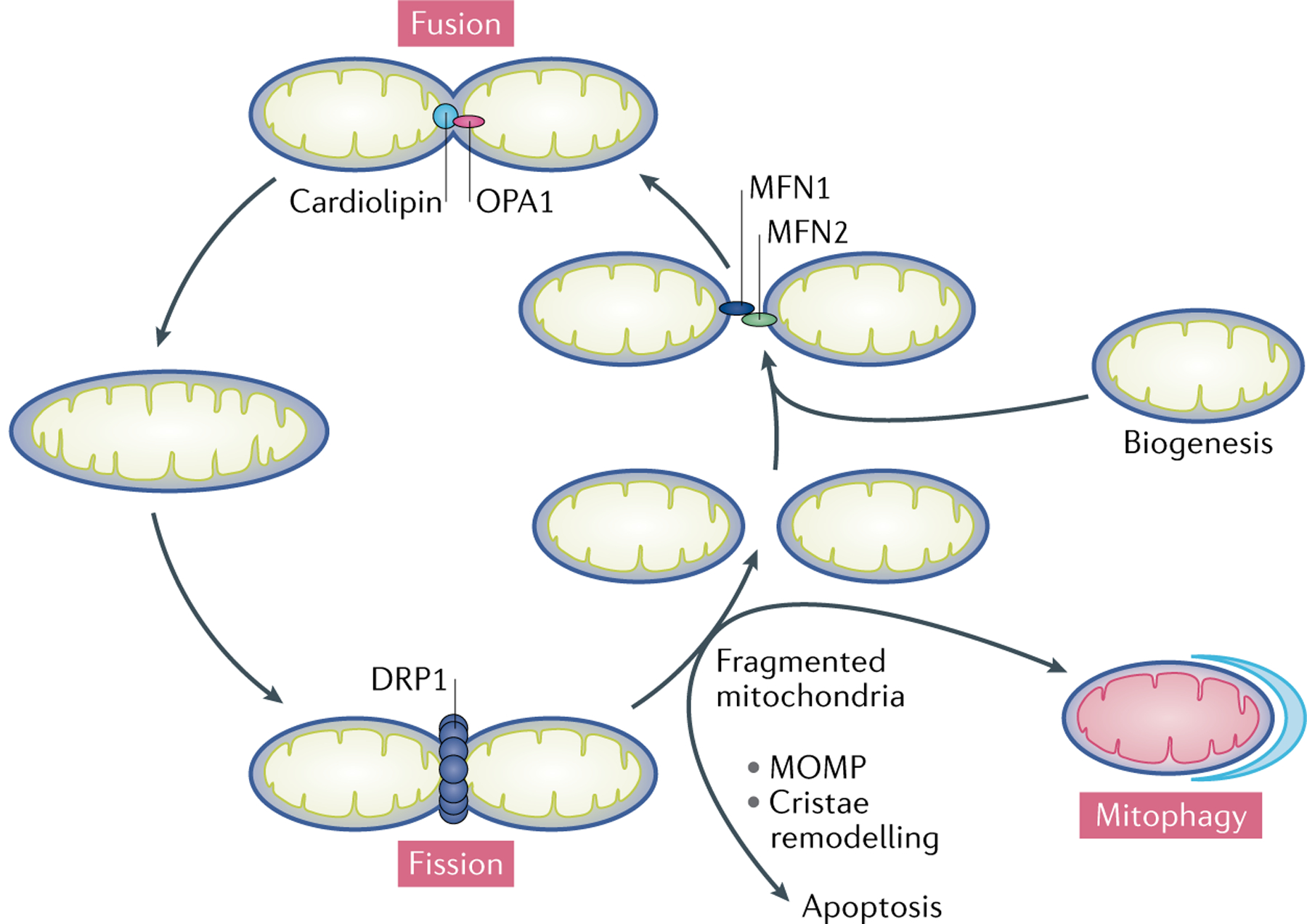
During fusion, mitofusin 1 (MFN1) and MFN2 expressed on two adjacent mitochondria interact to tether the organelles. GTP hydrolysis-induced conformational changes in the MFNs drive the docking and contact of the outer mitochondrial membranes (OMMs). The MFNs then oligomerize to fuse the OMMs. Following OMM fusion, inner mitochondrial membrane fusion is mediated by dynamin-like 120 kDa protein, mitochondrial (OPA1), which interacts with cardiolipin. Mitochondrial fusion facilitates the exchange of metabolites and substrates between mitochondria to ensure optimal functioning of the mitochondrial network and is also required for the complementation of damaged mitochondrial components to mitigate mitochondrial stress. During fission, dynamin-1-like protein 1 (DRP1) is recruited from the cytosol to the mitochondria, where it oligomerizes to form a ring-like structure around the OMM that utilizes the energy from GTP hydrolysis to constrict the organelle. Mitochondrial fission is required to separate damaged or dysfunctional components of mitochondria for selective autophagic degradation via mitophagy. Mitochondrial fragmentation, as a result of excessive mitochondrial fission over fusion, leads to mitochondrial outer membrane permeabilization (MOMP) and/or cristae remodelling, ultimately resulting in cell death.
