Figure 2.
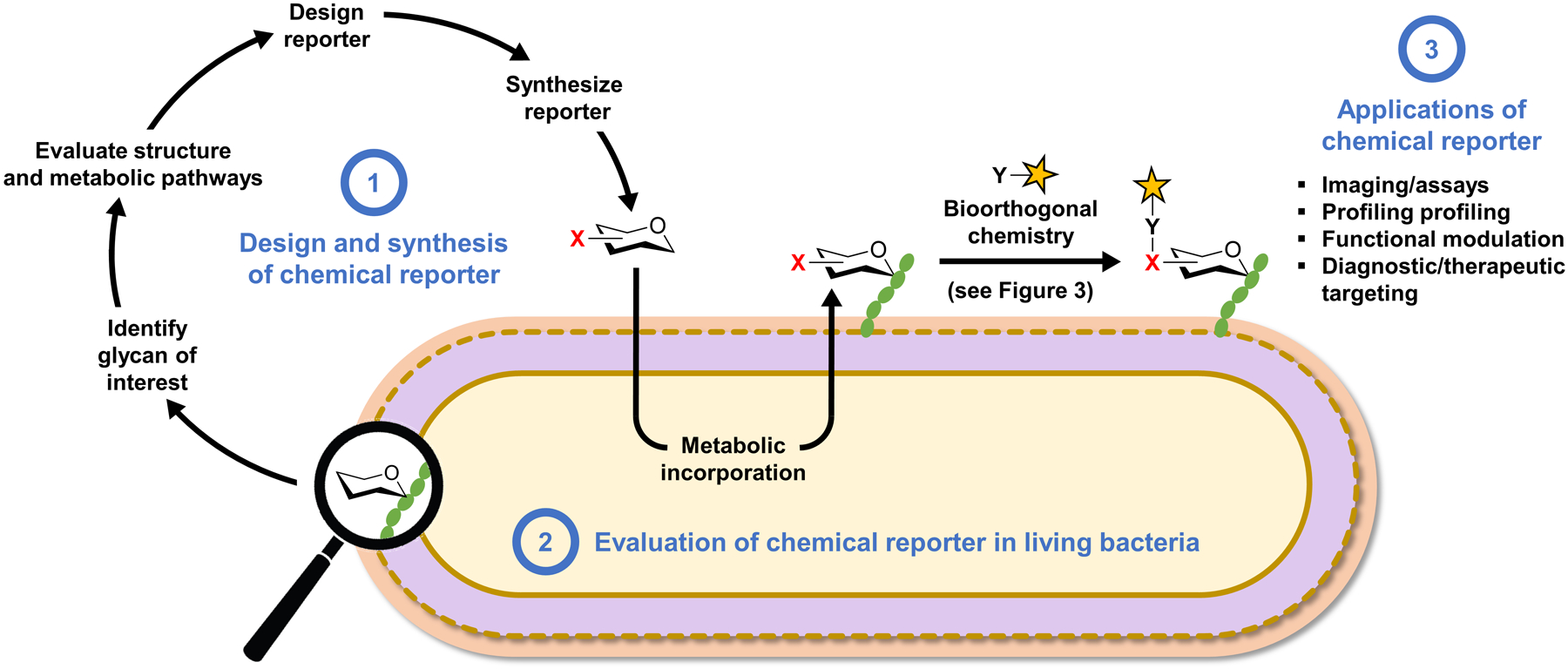
Overview of the bioorthogonal chemical reporter strategy as applied to studying bacterial glycans and workflow for the development of chemical reporters. The chemical reporter (shown as a hexose ring with a bioorthogonal functional group X) is fed to a living bacterial cell and metabolically incorporated into a glycan of interest. Next, the X-labeled glycan is reacted with an exogenously delivered reagent (e.g., a fluorophore) containing a complementary bioorthogonal functional group Y. A highly selective bioorthogonal reaction takes place on the cell surface between X and Y, leading to covalent ligation of the delivered cargo to the glycan of interest, enabling its analysis or modulation. See Figure 3 for common bioorthogonal reactions. In the one-step metabolic incorporation approach, X is typically a fluorophore and does not require a subsequent bioorthogonal reaction. The development of chemical reporters involves three stages (highlighted in blue text): (1) the reporter molecule must be designed and synthesized after selection of the target glycan and analysis of its structure and biosynthesis; (2) next, the reporter must be tested in bacterial cells to determine whether it successfully labels the glycan of interest and to elucidate the pathway of incorporation; (3) once the reporter’s behavior in cells is established, it can potentially be used for a variety of applications, ranging from live-cell imaging of glycan biosynthesis and dynamics to targeting the bacterium with therapeutic or diagnostic cargo within a host.
