Figure 35.
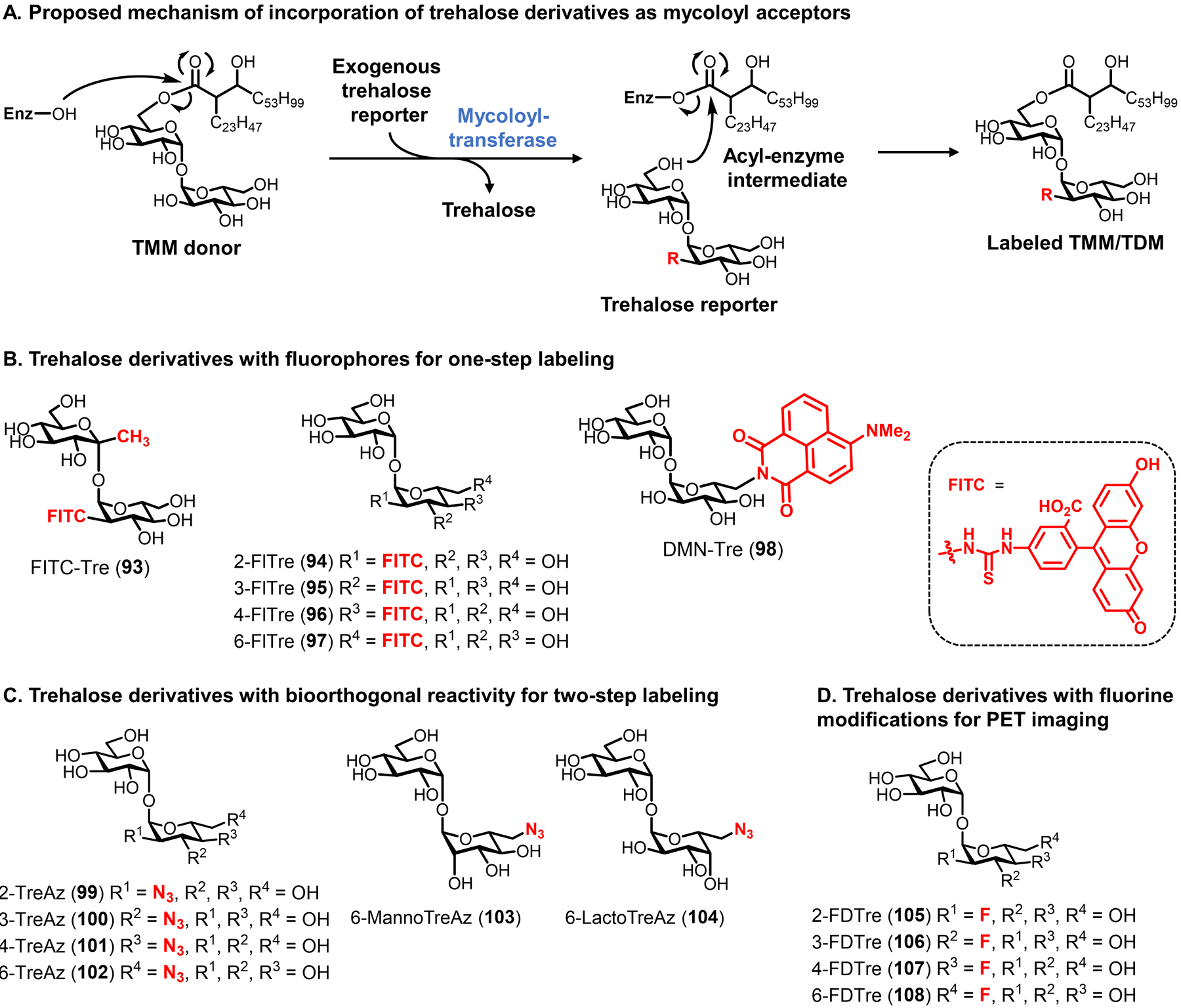
Trehalose derivatives for labeling trehalose mycolate glycolipids in mycobacteria. (A) Metabolic incorporation of unnatural trehalose derivatives (modified with an unnatural R group) is proposed to occur through mycoloyltransferase-catalyzed mycoloyl group transfer. Periplasmic Ag85-catalyzed transfer of mycoloyl group from native TMM to exogenous trehalose derivative is shown. A similar mechanism would occur for cytoplasmic Pks13-mediated mycoloyl group transfer onto intracellular trehalose derivatives. Depending on the position of the unnatural modification, mycoloyl groups could add to one 6-position to give a labeled TMM or both 6-positions to give a labeled TDM. Enz, enzyme with mycoloyltransferase activity (e.g., Ag85). (B–D) Structures of some published trehalose derivatives bearing fluorophores (B), bioorthogonal functional groups (C), and fluorine modifications (D).
