Figure 37.
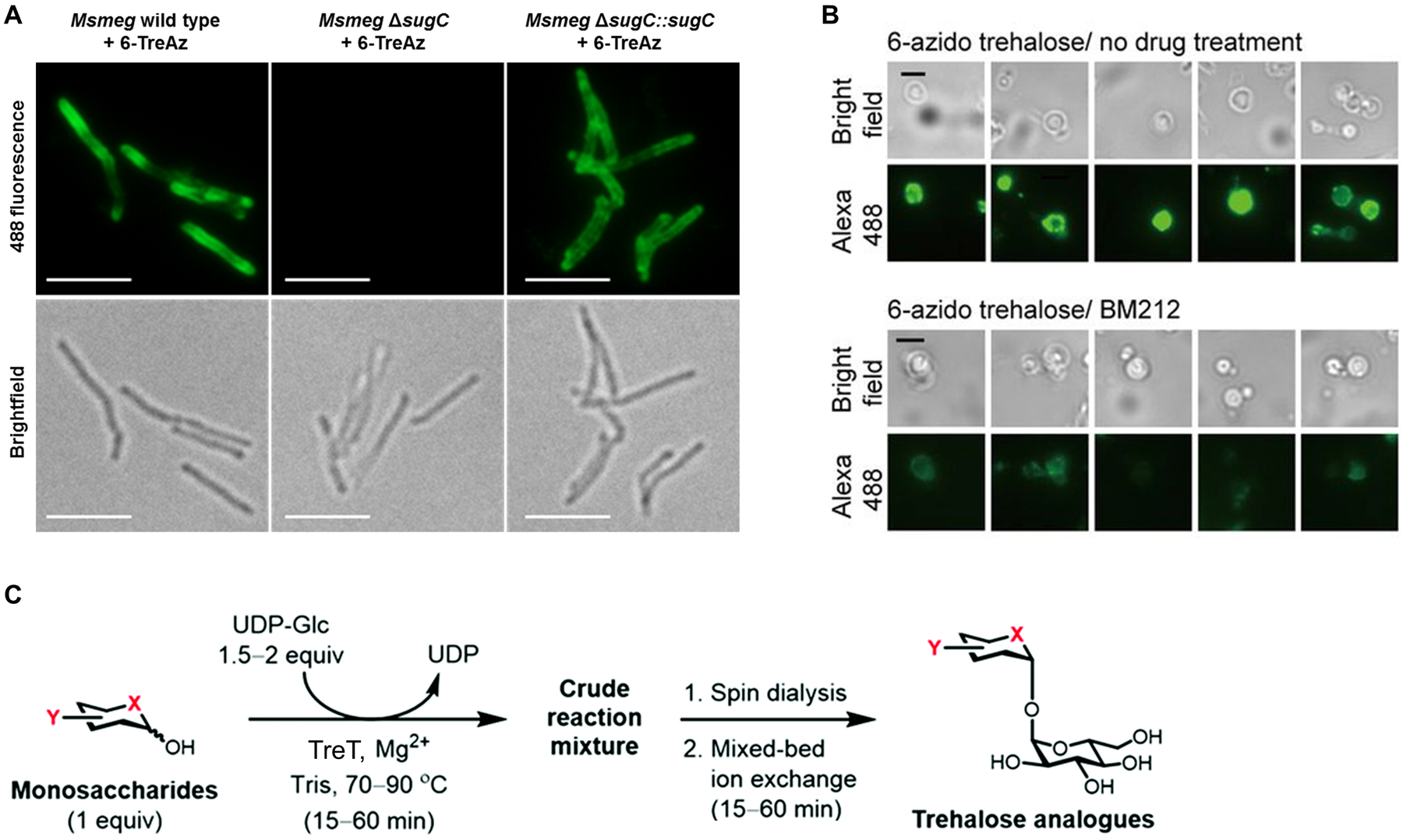
Labeling trehalose mycolates in mycobacteria using azido trehalose (TreAz) derivatives. (A) M. smegmatis (Msmeg) wild type, ΔsugC mutant lacking the trehalose transporter, or ΔsugC::sugC complement with transporter restored were incubated in the presence of 25 μM 6-TreAz (102), fixed, reacted with alkyne-488 via CuAAC, and imaged by fluorescence microscopy. Top, 488 channel; bottom, brightfield channel. Scale bars, 5 μm. Reproduced with permission from ref 306. Copyright 2014 Wiley-VCH. (B) M. smegmatis spheroplasts were incubated with 100 μM 6-TreAz (102) in the absence (top) or presence (bottom) of MmpL3 inhibitor BM212, reacted with cyclooctyne-biotin via SPAAC, stained with 488-streptavidin, and imaged by fluorescence microscopy. Scale bars, 3 μm. Reproduced from ref 307. (C) TreT-catalyzed one-step chemoenzymatic synthesis and all-aqueous purification of trehalose derivatives. Various unnatural acceptor substrates can be converted into products, with Y representing azido-, deoxy-, fluoro-, or stereochemical modifications, and X representing oxygen or sulfur atoms. Figure adapted from ref 297 with permission from the Royal Society of Chemistry.
