Figure 7.
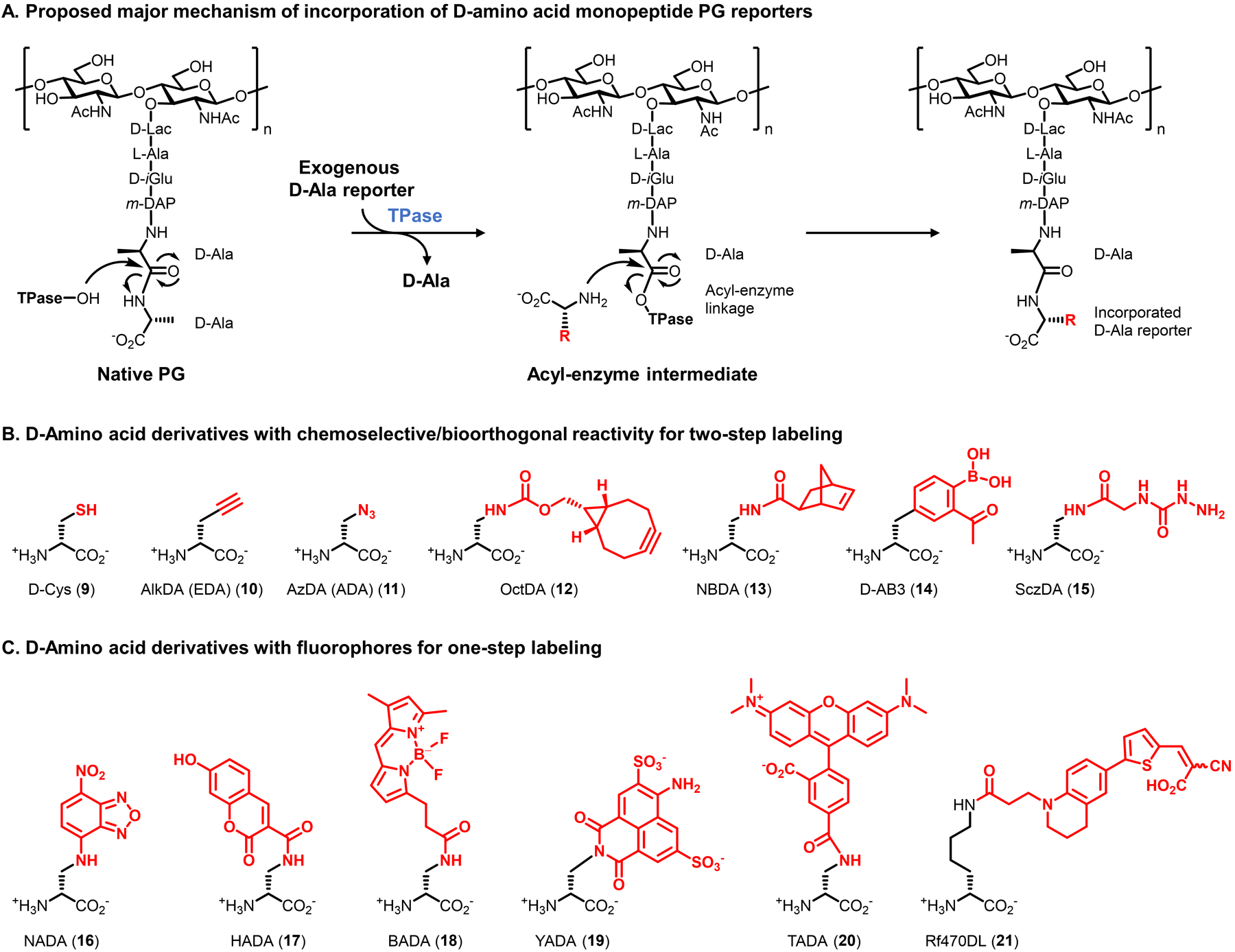
D-amino acid derivatives targeting the PG stem peptide. (A) Metabolic incorporation of unnatural D-amino acid derivatives (modified with an unnatural R group) is proposed to predominantly occur through periplasmic TPase-mediated PG remodeling. Incorporation by PBP D,D-TPases to install the probe at the 5-position of the peptide is shown; incorporation by L,D-TPases to install the probe at the 4-position can also occur. Smaller D-amino acid derivatives may additionally incorporate into PG via an intracellular route. (B and C) Structures of D-amino acid derivatives bearing selectively reactive functional groups (B) and fluorophores (C).
