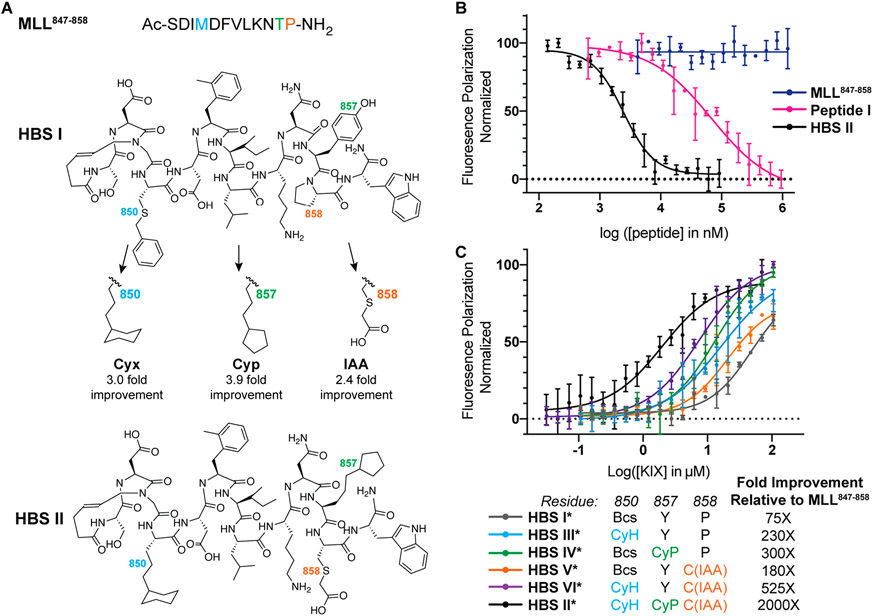
Figure 5.
Analysis of the impact of nonnatural side chains on the binding affinities of MLL peptides. (A) The fragment screen yielded optimized peptide HBS II from HBS I as a starting point. (B) HBS II binds KIX with submicromolar affinity, which corresponds to a 2000-fold improvement over MLL847–858 and a 50-fold improvement over the computationally optimized Peptide I. (C) HBS helices III*–VI* with single and double side-chain fragment hits were evaluated. Each nonnatural appendage offers cumulative enhancement to peptide affinity. *Denotes fluorescently labeled peptide. The binding constants for the peptides are listed in Table S2.