Figure 2.
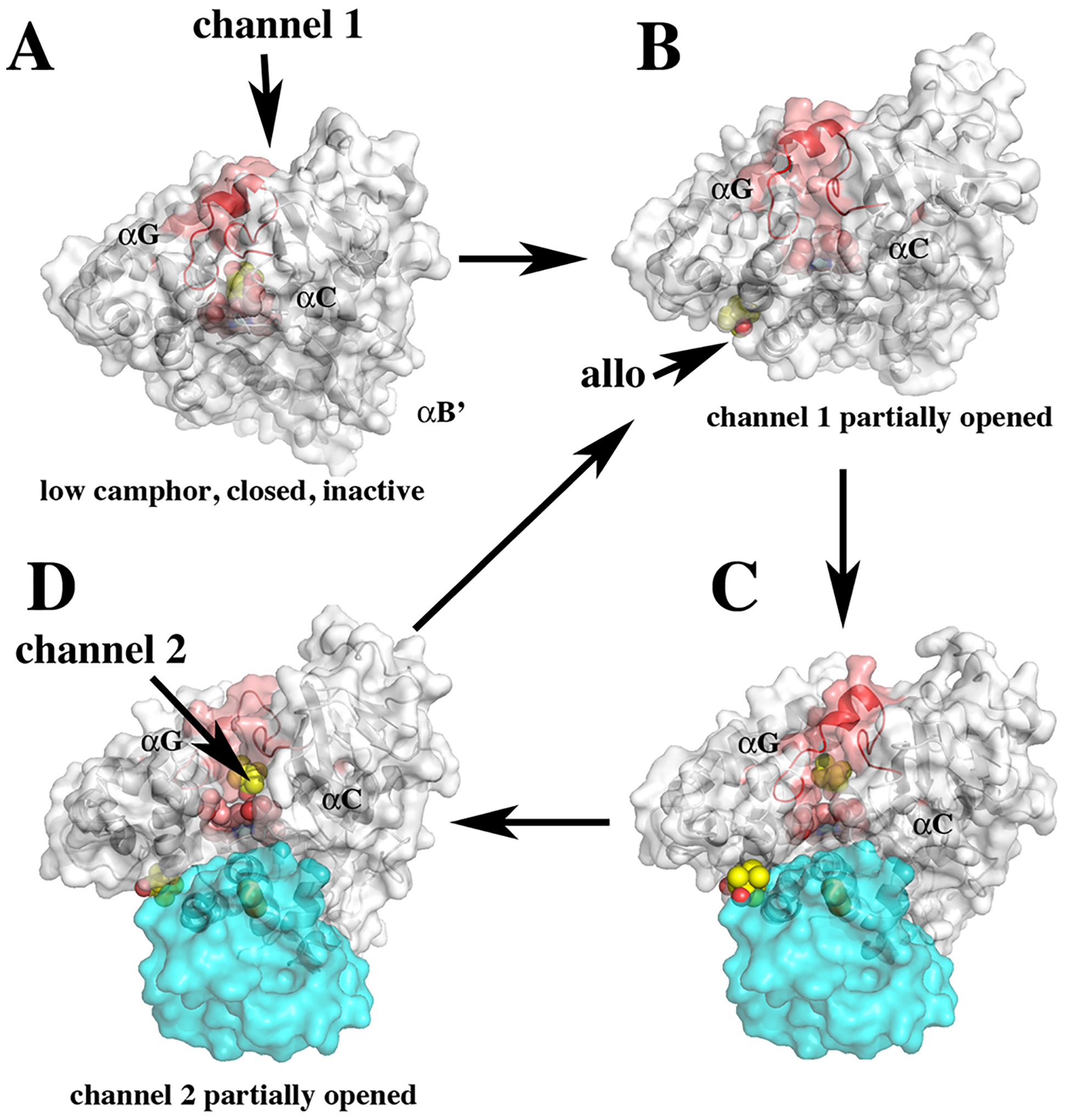
Various conformational states consistent with crystal structures, NMR, and molecular dynamics. (A) At low-camphor concentrations, camphor is bound in the active site and P450cam is in the closed inactive state. (B) At higher camphor concentration, the allosteric site is occupied, channel 1 opens, and Pdx binds (C). These events destabilize the salt bridges to Asp251, which triggers the formation of the proton relay network required for O2 activation. (D) Product forms and channel 2 opens, thereby enabling product egress. The B′ helix provides key contacts with the substrate. Helix F that undergoes the large open/close motion is labeled.
