Figure 3.
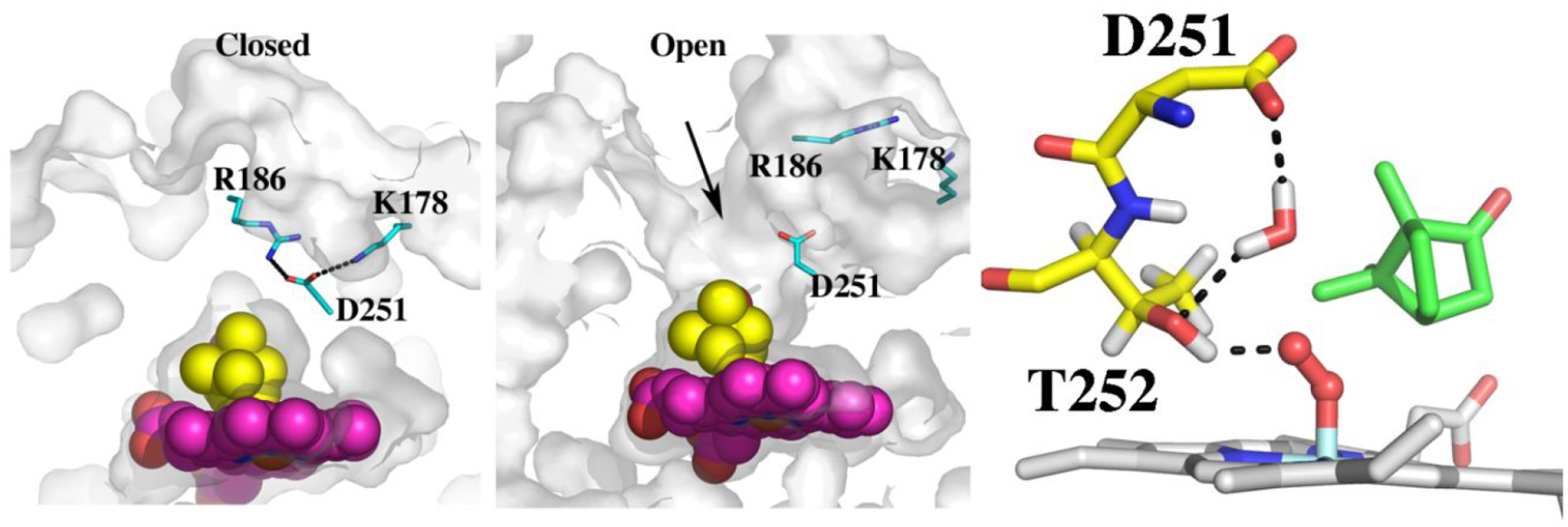
Crystal structures of the closed (2CPP) and open (4JX1) forms of P450cam. In the closed state, Asp251 is tied up with salt bridges to Arg186 and Lys178. When Pdx binds, these salt bridges are broken and the active site opens. The arrow indicates where a second camphor molecule is observed in 3 of the 4 asymmetric units in 4JX1. Also shown is the proposed proton relay network taken after Ugur et al.21 Thr252 is the direct proton donor to the iron-linked dioxygen while a water bridges between Asp251 and Thr252. For Asp251 to shuttle protons into the active site it must be able to adopt different rotamer conformations. The structure of the P450cam–Pdx complex22 in the presence of cyanide which mimics the oxy complex shows that Asp251 can, indeed, adopt the “in” rotamer as shown.
