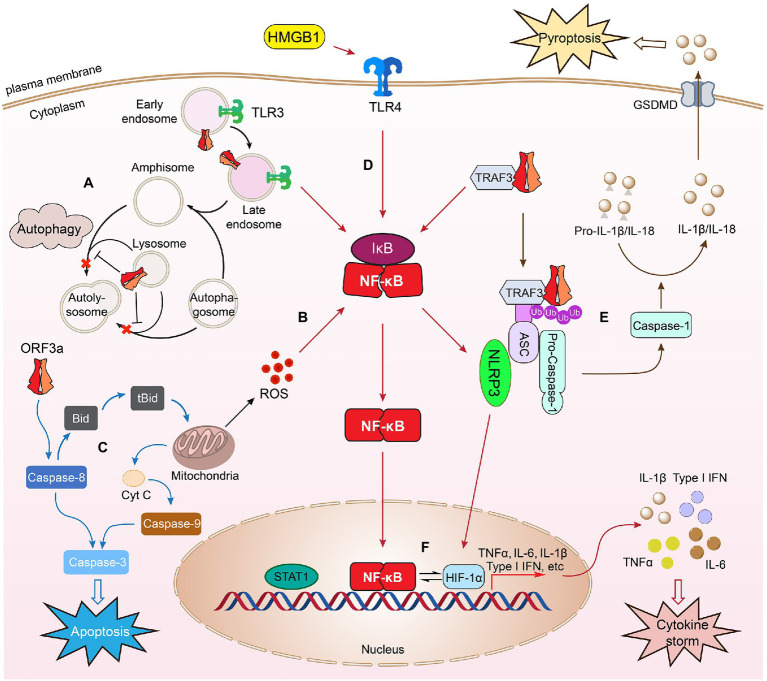
Figure 4.
SARS-CoV-2 ORF3a–host cell interaction. Expression of SARS-CoV-2 ORF3a in infected cells triggers cellular innate immune responses such as autophagy (A). Autophagy is an important cellular antiviral response to trap viruses in autophagosomes for lysosomal degradation. An autophagosome with trapped virus fuses with an endosome to generate an amphisome, which then fuses with lysosomes, forming an autolysosome, where virus cargos can be recycled by lysosomal enzymes. ORF3a counteracts cellular autophagy activity by blocking fusion of autophagosomes/amphisomes with lysosomes. ORF3a also triggers cellular oxidative stress response leading to increased ROS production (B) and induces mitochondria-dependent and mitochondria-independent apoptosis (C). SARS-CoV-2 ORF3a induces cellular pro-inflammatory immune responses to activate cytokine (TNFα, IL-6, and IFN-1β) production through NF-κB, TLR4, or TLR3-mediated process (D). ORF3a interacts with TRAF3 triggering downstream NF-κB activation and cytokine induction leading to activation of NLRP3 inflammasomes. Formation of the NLRP3 inflammasome activates caspase-1 that converts pro-IL-1β to IL-1β through a proteolytic processing leading to pyroptosis (E), which is an inflammatory form of lytic cell death that promotes rapid clearance of invading viruses and enhances host antiviral response (Jorgensen and Miao, 2015). ORF3a-induced NF-κB activation could activate NLRP3 inflammasomes, which in turn activates HMGB1 and HIF-1α to promote the production of pro-inflammatory cytokines and possibly induction of cytokine storm (F). As a result, it induces cell death through apoptosis, necrosis, and pyroptosis, leading to tissue damage, COVID-19 or post-COVID conditions. This figure is generated using Adobe Illustrator 2020.