Abstract
The rapid emergence and spread of antimicrobial resistance has become a global public health concern that threatens the effective treatment of infectious diseases. One major approach adopted to overcome antimicrobial resistance is the use of plant extracts individually and/or with combination of antibiotics with plant extracts, which may lead to new ways of treating infectious diseases and essentially representing a potential area for further future investigations. In this study, the antifungal activities of Azadirachta indica leaf and Catharanthus roseus flower extracts against fluconazole-resistant Candida albicans strains (isolated from pregnant women with vulvovaginal candidiasis) and anti-methicillin-resistant Staphylococcus aureus (MRSA) were evaluated by agar well diffusion, microdilution, and biofilm inhibition assays. Subsequently, the determination of the combined antimicrobial activity of the individual plant extracts with (fluconazole and voriconazole) and (ampicillin, tetracycline, and streptomycin) against C. albicans strains and MRSA, respectively, was evaluated by checkerboard microdilution assay. Results from the study showed that the antimicrobial activity of the two plant extracts determined by time-kill kinetics was fungistatic with their MICs ranging from 0.1 to 4 mg/mL. Interestingly, all extracts were proved as good biofilm inhibitors of resistant C. albicans and MRSA from 10.1 to 98.82%. Their combination interaction with fluconazole, voriconazole, ampicillin, tetracycline, and streptomycin ranged from synergy to antagonism as per the parameters used. Overall, these results showed that A. indica leaf and C. roseus flower extracts have significant antifungal property. Furthermore, A. indica leaf and C. roseus flower extracts alone or in combination with fluconazole and voriconazole could provide a promising approach to the management of candidiasis caused by drug-resistant strains as well as their interaction with the antibacterial agents to combat the common infections caused by MRSA.
1. Introduction
Antimicrobial resistance is of grave concern globally since it threatens the gains made over the years to reduce the prevalence of infectious diseases, most especially in Africa [1]. It is responsible for about 700,000 deaths worldwide with a greater percentage of this death happening in low- and middle-income countries [2]. The development of resistance mechanisms such as biofilm formation, efflux pump possession, target site diversion, and resistance gene acquisition among others by bacterial species to inactivate the effectiveness of most antibacterial agents has become a major health security threat to the medical world in recent times [2, 3]. Among these mechanisms, biofilms have been noted to form 65% of microbial infections and bacteria living in them develop resistance to antibiotics a thousand times than those existing as planktonic cells, thereby calling for urgent attention [4]. Recent studies have proven that about 95% of Staphylococcus aureus are now resistant to penicillin as well as methicillin given to patients in hospitals and communities [5]. Methicillin-resistant S. aureus (MRSA) is one of the common causes of multidrug resistance infections with significant morbidity and mortality [6]. This has been attributed to the abuse of over-the-counter (OTC) medications most especially without prescription [7]. In many clinical settings, recurrent candidiasis has been treated mostly by administering standard antifungal drugs, such as azoles, polyenes, and echinocandins. However, in recent times, reports have shown that there has been an increase in the number of resistant Candida species especially C. albicans against most of these standard drugs [8, 9].
The resistance of Candida species to many of these antifungal agents has been attributed to the formation of Candida biofilms, which can occur on mucosa or endothelia surfaces as well as medical devices such as indwelling joint prostheses, cardiac valves, and intravascular and urinary catheters [10, 11].
As such, the intervention adopted to combat this menace has been the use of combination therapy either with orthodox medications or plant-based products. Because these combination drugs have been shown to have the ability to modulate the resistance capacity of these microbial organisms [12, 13]. Despite these gains in the fight against antibiotic resistance, there still remains much more to be done in search of new drugs which are efficacious to help curb the ever-increasing antimicrobial resistance menace. Natural products and their plant derived compounds are mostly a source of new drug agents as well as lead compounds with little or no side effects [14]. According to the WHO, about 65% of the world's population rely on the use of herbs for their primary health care needs most especially in the sub-Saharan Africa region [15]. Azadirachta indica and Catharanthus roseus are two plants that possess antimicrobial activities are used traditionally for various infectious disease conditions in Ghana.
Neem plant (Azadirachta indica A. Juss) is a tree belonging to the family Meliaceae. The different parts of the plant are used as antiseptic, diuretic, and in the management of diseases such as, cough, nausea, vomiting, fever, and peptic ulcer [16]. Catharanthus roseus (L. G. Don) from the family Apocynaceae contributes vastly to the treatment of several diseases including cancer. It is an evergreen herbaceous plant native to Madagascar [17]. A study on this plant has shown that the ethanol leaves extract of C. roseus showed antimicrobial activity against Staphylococcus aureus, Escherichia coli, and C. albicans [18, 19].
This study therefore sought to investigate the antimicrobial, antibiofilm, and resistance modulatory potentials of C. roseus and A. indica on biofilm forming fluconazole-resistant C. albicans and MRSA.
2. Methods
2.1. Experimental Plant Materials
The plant materials A. indica leaves and C. roseus flowers were collected from the campus of the University of Health and Allied Sciences, Ho Municipality. The plants were identified, and voucher specimens were deposited in the Herbarium of the Institute of Traditional and Alternative Medicine, University of Health and Allied Sciences, Ho, Ghana, with a voucher number (UHAS/ITAM/2020/L002 and UHAS/ITAM/2020/L003).
2.2. Test Organisms
Four fluconazole-resistant Candida albicans isolates were obtained from the Department of Medical Microbiology, Ho teaching Hospital, Ghana. These strains were primarily isolated from pregnant women with vulvovaginal candidiasis. To guarantee the purity of the Candida isolates, separate yeast colonies were subcultured on Sabouraud Dextrose Agar (SDA) (Oxoid Ltd., Hampshire, UK), with chloramphenicol and incubation at 37°C for 24–48 h. Subsequently, presumptive identification of C. albicans was carried out by examination of colony morphology, microscopic examination of Gram-stained preparations, production of chlamydospore, germ tube test, and sugar fermentation tests [20]. Confirmation with API ID 32C strips was done using standard microbiological procedures (BioMerieux, France). Additionally, methicillin-resistant Staphylococcus aureus (NCTC 29212) used in this study was obtained from the Microbiology Laboratory, Department of Biomedical Sciences, School of Basic and Biomedical Sciences, UHAS, on the basis of its implication in most infections.
2.3. Detection of Susceptibility to Fluconazole
Antifungal susceptibility testing of fluconazole (25 μg) (Oxoid Ltd., Basingstoke, UK) was carried out by disc diffusion method as described by Clinical and Laboratory Standards Institute (CLSI, document M44-A2, 2009). A zone diameter of ≥19 mm was considered sensitive, 15 to 18 mm was considered dose-dependently susceptible, and a diameter ≤14 mm was considered resistant [21].
2.4. Extraction and Preparation of Plant Materials
Each of the plant parts of A. indica and C. roseus washed under running tap water were air-dried at room temperature (25 to 32°C) for one to two weeks [22]. The dried plant materials were milled using a laboratory warring blender into coarse powder. Each processed plant material (40 g) was cold macerated with 200 mL of 70% v/v ethanol for two days with intermittent stirring. The mixture was then filtered with a Whatman paper No. 1 and filtrate was evaporated to dryness using rotary evaporator under reduced pressure at 38°C, oven-dried at 40°C, and stored in a fridge at 4°C until use.
2.5. Phytochemical Screening
Presence of alkaloids, flavonoids, steroids, terpenoids, saponins, tannins, and glycosides were tested as per the method described by Visweswari et al. [23] and Neglo et al. [24] with slight modification. Grading of the final reaction of the secondary metabolites was done by comparing the results obtained for the plant extracts using how deep or light the color change was seen. Table 1 shows a summary of the various tests that were conducted.
Table 1.
| Phytochemical | Test | Observation |
|---|---|---|
| Alkaloid | Wagner's reagent (I2/KI) was used. Minute quantity of extracts was dissolved in dilute HCl and filtered. Few drops of Wagner's reagent (I2/KI) were added to about 2 mL of the filtrate. | Formation of brownish/red precipitate was used to determine the presence of alkaloid. |
|
| ||
| Flavonoids | Sulphuric acid (H2SO4) test was done by treating a fraction of the extract with concentrated H2SO4. | Formation of orange color was used to detect the presence or absence of flavonoids. |
|
| ||
| Steroids | Liebermann–Burchard test was used. Four milligrams of the extracts was treated with 0.5 ml of acetic anhydride and 0.5 mL of acetic acid. Concentrated H2SO4 was slowly added. | The development of a reddish/brown color indicated the presence of steroids. |
|
| ||
| Terpenoids | Liebermann–Burchard test was used. Four milligrams of the extracts was treated with 0.5 ml of acetic anhydride and 0.5 mL of acetic acid. Concentrated H2SO4 was slowly added. | The development of a blue-green color indicated the presence of terpenoids. |
|
| ||
| Saponins | This was tested for using foam test. Exactly 0.5 g of the plant extract was dissolved in 2.5 mL of distilled water. The mixture was shaken vigourously. | The presence of foam indicated the presence of saponins. |
|
| ||
| Tannins | Ferric chloride test was used to test for the presence of tannins. An exact amount of 0.5 g of the extract was boiled in 20 mL of distilled water and filtered afterwards. Few drops of 0.1% of FeCl3 were added. | The presence of brownish-green, brownish-black, or blue-black color was used to detect the presence of tannins. |
|
| ||
| Glycosides | Benedict's test was used for the detection of glycosides. Precisely, 0.5 g of plant extract was dissolved in 5 ml of distilled water. Exactly 2 mL of Benedict's solution was heated and 8 drops of the dissolved sample were added and allowed to boil for 5 minutes. | Formation of brick-red precipitate indicated the presence of glycosides. |
2.6. Determination of Antifungal Activity of the Plant Materials
The antifungal activity of the extracts was determined using both the Kirby–Bauer agar well diffusion method and the broth microdilution method [25].
2.7. Agar Well Diffusion
20 mL of sterile Muller–Hinton agar was poured and allowed to set and then inoculated with 100 μl of 1x 106 colony-forming units (CFU)/mL of MRSA. All the strains (MRSA and C. albicans) were cultured overnight and grown at 37°C in Muller–Hinton broth with further dilution to 0.5 McFarland standards with saline and then inoculated on Muller–Hinton agar. Five wells were bored in each plate using a cork borer (No. 3, 5 mm). These wells were filled with 100 μl of 10, 20, and 40% of ethanol extracts of the two plant materials. 20% DMSO was used as a negative control, whereas tetracycline (10 μg/disc) and voriconazole (25 μg/disc) were used as positive control for MRSA and C. albicans inoculum, respectively. Each of the extracts were then allowed to diffuse for 15 mins at room temperature after which they are incubated at 37°C for 48 h and zones of inhibitions were recorded. The procedure was performed in triplicate.
2.8. Minimum Inhibitory Concentrations of the Extracts and the Standard Antibiotics/Antifungal Agents
The minimum inhibitory concentrations (MICs) of the extracts and the referenced drugs (ampicillin, tetracycline, and streptomycin fluconazole and voriconazole dissolved in sterile distilled water except tetracycline in 10% ethanol) were carried out according to the method described by [25] with slight modification.
Briefly, each well of a 96-well microtitre plates was filled with 150 μL of the double-strength Mueller–Hinton broth. This was followed by dilutions of each of the extracts and the referenced drugs ranging from 0.063 to 256 mg/mL and 0.063 to 128 μg/mL by adding 150 μL of each test sample for both the extracts and the drugs, respectively. In all cases, one well served as positive control inoculated with each test microorganism and the broth stock in the test tube as the negative control without organism. Afterwards, 150 μL of 106 cfu/mL of each test microorganisms prepared in the broth was added to each well.
The microtitre plates were then subjected to incubation at 37°C for 24 hours, after which 40 μL of 3-(4,5- dimethylthiazole-2- yl)-2,5-diphenyltetrazolium bromide (MTT) (0.2% w/v) was added to each well. The plate was incubated at 37°C for 30 min, and the appearance of purple color signified growth. The concentration at which the extracts and the referenced drugs did not show any change in color was noted as the minimum inhibitory concentration.
2.9. Determination of Synergistic Effect of Test Plant Samples and Selected Antimicrobial Agents
In-vitro analysis of the interaction between test samples from A. indica and C. roseus and (i) antibiotics (ampicillin, tetracycline, and streptomycin) against MRSA (NCTC 19243) and (ii) antifungals (fluconazole and voriconazole) against C. albicans strains were evaluated by adopting checkerboard microdilution assay as described previously by Khodavandi et al. [26] and Dickson et al. [27] with slight modifications. The tested concentrations for each ampicillin, tetracycline, streptomycin, fluconazole, and voriconazole and 0.5 mg/ml subinhibitory concentrations of each test plant sample ranged from 0.063 to 64 μg/mL.
The mode of the interactions was measured by calculating the fraction inhibitory concentration index (FICI). The FICI was estimated by
| (1) |
where Ac is the MIC of antibiotic/antifungal in combination, Ec is the MIC of each test plant sample in combination, Aa is the MIC of each antibiotic/antifungal alone, and Ea is the MIC of each test plant sample alone. The interaction was considered synergistic if the FICI was ≤0.5, partial synergistic if FICI was >0.5 and <1, additive if FICI was = 1, no difference if the FICI was >1 and ≤4, and antagonistic if the FICI was >4.0.
2.10. Formation of Biofilms by the Clinical C. albicans Strains and MRSA (NCTC 19243)
The biofilm-forming potentials of the clinical C. albicans cultures and clinical standard MRSA (NCTC 19243) diluted to 0.5 McFarland standard in Mueller–Hinton broth were employed per the protocol described by Haque et al. [28] and Neglo et al. [24] with slight modification. Briefly, 200 μL of each of the standardized inoculum prepared was added to 2 mL of broth in test tubes and incubated at 37°C for 24 and 48 hours with regard to bacteria or fungi, respectively.
Planktonic cells were aspirated and washed from the tubes to get rid of floating cells; the tubes were then dried at 25–28°C in incubator and stained with 2 mL of 0.1% crystal violet for 15 mins after which they were washed with sterile water and further dried at room temperature. The adherent microbial biofilms on the walls of the tubes were reconstituted with 2 mL ethanol and the absorbance of each sample was read at 595 nm with a UV spectrophotometer (Jenway, Bibby Scientific Ltd., Stone, Staffordshire, UK).
The optical density (OD) of the sterile broth was subtracted from that of the microbial biofilms formed to compensate for the background absorbance. Each of the procedures was performed in triplicate.
2.11. Biofilm Inhibition Effects of the Extracts
The potential of the various extracts to inhibit biofilm formation was determined as per the protocol described by Haque et al. [28] and Alshami and Alharbi [29]. One ml of each of the extracts was diluted with 1 ml of the Mueller–Hinton broth in sets of test tubes arranged to arrive at concentrations ranging from 32 mg/ml to 1 mg/ml, after which 10 μl of each of the standardized MRSA containing 105 per mL microorganisms was added. Control wells containing no extracts were included as well.
Each of the tubes was then incubated undisturbed at 37°C for 48 hours. The planktonic cells were aspirated and washed from the tubes and subsequently dried at 25–28°C in incubator, after which they were stained with 1 mL of 0.1% crystal violet for 15 mins. Each tube was further washed with sterile water and dried at room temperature. The adherent microbial biofilms on the walls of each tubes were reconstituted with 1 mL ethanol and the absorbance of each sample read at 595 nm with a UV spectrophotometer (Jenway, Bibby Scientific Ltd., Stone, Staffordshire, UK) after being blanked, and the optical density of the culture media control was subtracted to obtain inhibitory effects of the extracts. Each of the procedures was performed in triplicate.
The biofilm inhibition potential of each of the extracts to reduce the optical density compared to the negative control was noted as the biofilm inhibitory activity.
| (2) |
2.12. Time-Kill Kinetics Assay of the Extracts
The time-kill kinetics of the various extracts were carried as per the protocol designed by Appiah et al. [30] with slight modification. Briefly, the microbial strain MRSA (NCTC 19243) and clinical C. albicans strains were standardized to 106 cfu/mL cell concentration in test tubes. Subsequently, concentrations equal to the MIC, twice the MIC, four times and 8 times MIC of each extract as in Tables 2 and 3 were prepared and mixed with sterile broth in test tubes. Each of the tested microorganisms is then added and incubated at 37°C. Aliquots of 1 ml of each of the various medium were pipetted into well-labelled Petri dishes at intervals of 0, 6, 18, 30, 54, and 72 h for the individual tested organisms. Nutrient agar was then poured onto each medium transferred and incubated at 37°C for 24 h. Inoculums of each of the strains were treated similarly alongside as the control. The colony-forming unit of the test organisms was determined and the experiment was carried out in triplicate for each. Graphs of log CFU were plotted against time for each treatment and the data obtained from the study was analysed using one-way ANOVA followed by post hoc test using Graph Pad Prism Version 5.0.q.
Table 2.
Synergistic effect of A. indica and C. roseus on fluconazole and voriconazole against resistant C. albicans strains.
| Test sample | Fluconazole | Interpretation | Voriconazole | Interpretation |
|---|---|---|---|---|
| FIC index | FIC index | |||
| A. indica | ||||
| CA1 | 0.13 | Synergy | 16.13 | Antagonism |
| CA2 | 5.00 | Antagonism | 1.5 | No difference |
| CA3 | 1.00 | Additive | 1.00 | Additive |
| CA4 | 0.13 | Synergy | 0.63 | Partial synergy |
|
| ||||
| C. roseus | ||||
| CA1 | 5.25 | Antagonism | 37.00 | Antagonism |
| CA2 | 1.00 | Additive | 1.00 | Additive |
| CA3 | 2.07 | No difference | 2.17 | No difference |
| CA4 | 0.63 | Partial synergy | 0.63 | Partial synergy |
Table 3.
Synergistic effect of A. indica and C. roseus on selected antibacterial agents against MRSA.
| Test sample | Ampicillin | Interpretation | Tetracycline | Interpretation | Streptomycin | Interpretation |
|---|---|---|---|---|---|---|
| FIC index | FIC index | FIC index | ||||
| A. indica | ||||||
| MRSA | 0.500 | Synergy | 0.500 | Synergy | 2.50 | No difference |
| C. roseus | ||||||
| MRSA | 8.50 | Antagonism | 0.75 | Partial synergy | 2.50 | No difference |
2.13. Statistical Analysis
All measurements are expressed as mean ± standard error mean (SEM) or mean ± SD of independent experiments. IC50 values were obtained by interpolations from standard curves. All tests were carried out in triplicate.
3. Results
3.1. Preliminary Phytochemical of the Extracts
The phytochemical results obtained for the ethanolic extracts are presented in Table 4. The alkaloids, reducing sugars, and saponins are present in all the extracts in varied proportions while the flavonoids are absent in all the extracts. However, A. indica leaf and C. roseus flower extracts possessed a high-to-moderate level of the alkaloids with steroids being absent in them. A. indica leaf and C. roseus leaf extracts had high-to-low levels of reducing sugars; the tannins were low in A. indica leaf and absent in the other two extracts.
Table 4.
Phytochemical screening results of ethanolic extracts of Azadirachta indica and Catharanthus roseus.
| Phytochemical | Presence | |
|---|---|---|
| A. indica leaves | C. roseus flowers | |
| Alkaloids | + | + |
| Flavonoids | − | − |
| Steroids | − | − |
| Terpenoids | − | + |
| Saponins | + | + |
| Tannins | + | − |
| Reducing sugars | + | + |
Note: +: present; −: absent.
3.2. Antimicrobial Activity of Plant Extracts
The ethanol extracts of both A. indica and C. roseus had antimicrobial activities against all microbes employed in this study. At various concentrations (40, 20, and 10%), each of the extracts recorded significant antifungal activity against all the fluconazole-resistant C. albicans strains and MRSA (NCTC 12493) in the disc diffusion assay with varied zones of inhibition (Table 5). A. indica extract had the higher inhibitory activity against MRSA with a value of 19.33 ± 0.67 mm at 40% as compared to C. roseus.
Table 5.
Diameter of inhibition zone of ethanolic extracts of A. indica and C. roseus against clinical isolates fluconazole-resistant C. albicans strains and MRSA (NCTC 12493).
| Plants (ethanol extracts) | Conc. (w/v %) | Zone of inhibition (mm) (mean ± SEM) | ||||
|---|---|---|---|---|---|---|
| CA1 | CA2 | CA3 | CA4 | MRSA (NCTC12493) | ||
| AI | 40 | 17.33 ± 0.33 | 15.67 ± 0.33 | 15.33 ± 0.33 | 19.33 ± 0.33 | 19.333 ± 0.67 |
| 20 | 13.66 ± 0.33 | 11.67 ± 0.33 | 13.67 ± 0.33 | 13.00 ± 0.578 | 13.000 ± 0.58 | |
| 10 | 10.33 ± 0.33 | 6.33 ± 0.33 | 10.67 ± 0.33 | 10.67 ± 0.33 | 10.667 ± 0.33 | |
| CR | 40 | 16.00 ± 0.57 | 13.0 ± 0.58 | 16.67 ± 0.33 | 14.33 ± 0.333 | 17.667 ± 0.33 |
| 20 | 11.667 ± 0.33 | 10.67 ± 0.33 | 14.33 ± 0.33 | 12.33 ± 0.333 | 11.000 ± 0.58 | |
| 10 | 0.000 ± 0.00 | 9.33 ± 0.33 | 11.33 ± 0.33 | 9.67 ± 0.333 | 6.667 ± 0.67 | |
| Positive control (fluconazole, 25 μg) | NI | NI | NI | NI | — | |
| D/control (tetracycline, 10 μg) | — | — | — | — | — | 27.33 ± 1.200 |
| Negative control (20% DMSO) | NI | NI | NI | NI | NI | |
Values are shown in triplicate and represented as mean ± SEM. AI: Azadirachta indica, CR: Catharanthus roseus, CA1: Candida albicans 1, CA2: Candida albicans 2, CA3: Candida albicans 3, CA4: Candida albicans 4, and NI: no inhibition.
Similarly, A. indica recorded the highest inhibitory activity against all C. albicans strains, out of which CA 4 gave the highest value of 19.33 ± 0.33 at 40%, while C. roseus showed the least activity in all varied concentration, except against CA 3. Both controls, positive and negative, however showed no activity, showing the relevant efficacy of the test extracts.
3.3. MIC of Plant Extracts and Antimicrobial Agents
In the broth dilution assay, microbes in inoculums were reduced in a dose-dependent manner by the extracts (Table 6). The trend of activity was however in the same order as in the disc diffusion method. While both plant extracts had good MICs against all the fluconazole-resistant C. albicans strains, the MICs of the extracts varied from one C. albicans strain to another within the range 0.1–4 mg/mL. With the test antifungal agents, the MICs ranged from 4 to 16 μg/mL as shown in Table 6. Similarly, both plant extracts had good MICs against MRSA (NCTC 12493) at 1 mg/L, while the test antibiotic's MICs ranged from 8 to 32 μg/mL (Table 6).
Table 6.
Minimum inhibitory concentration of ethanolic extracts and antimicrobial agents against fluconazole-resistant C. albicans strains and MRSA (NCTC12493).
| MIC of plant ethanol extracts against test organisms | |||||
| Test organisms | AI (mg/L) | Test organisms | CR (mg/L) | ||
|
| |||||
| CA 1 | 4.0 | CA 1 | 0.1 | ||
| CA 2 | 0.1 | CA 2 | 1.0 | ||
| CA 3 | 0.5 | CA3 | 0.3 | ||
| CA 4 | 4 | CA 4 | 4.0 | ||
| MRSA | 1.0 | MRSA | 1.0 | ||
|
| |||||
| MIC of antimicrobial agents against test organisms | |||||
| Test organisms | Fluconazole (μg/mL) | Voriconazole (μg/mL) | Tetracycline (μg/mL) | Ampicillin (μg/mL) | Streptomycin (μg/mL) |
|
| |||||
| CA 1 | >64 | 4.0 | NE | NE | NE |
| CA 2 | >64 | 16.0 | NE | NE | NE |
| CA 3 | >64 | 8.0 | NE | NE | >64 |
| CA 4 | >64 | 4.0 | NE | NE | NE |
| MRSA | NE | NE | 32.0 | 16.0 | 8.0 |
AI: Azadirachta indica, CR: Catharanthus roseus, CA1: Candida albicans 1, CA2: Candida albicans 2, CA3: Candida albicans 3, CA4: Candida albicans 4, MIC: minimum inhibitory concentration, and NE: not evaluated.
3.4. Synergistic Effect of Test Plant Samples and Selected Antimicrobial Agents against MRSA
The interactions of the antifungal combinations of the extracts of A. indica and C. roseus with fluconazole and voriconazole as well as ampicillin, tetracycline, and streptomycin examined using slightly modified microdilution checkerboard method are summarised in Tables 2 and 3. A. indica exhibited a significant synergy with fluconazole against a resistant strain of C. albicans 1 and 4, then addictive interaction against C. albicans 3 and antagonism against C. albicans 2. On other hand, A. indica shows a partial synergy with voriconazole against C. albicans 4, addictive interaction against C. albicans 3, no difference in interaction against C. albicans 2 and antagonism interaction against C. albicans 1. Again, against MRSA, there was a synergy between ampicillin and tetracycline with A. indica and no difference in interaction with streptomycin. Concurrently from the study, while C. roseus with fluconazole demonstrated partial synergy against C. albicans 4, addictive against C. albicans 2, no difference against C. albicans 3, and antagonism against C. albicans 1 in combination with voriconazole as well, same synergistic antifungal activities were recorded against the C. albicans strains. Again, in the combination of C. roseus with ampicillin, tetracycline, and streptomycin against MRSA, there was antagonism, partial synergy, and no difference interactions noted, respectively.
3.5. Biofilm Inhibition Potential of Plant Extracts
The result obtained showed that each C. albicans strains possessed considerable amount of biofilm with their absorbances ranging from 0.858 ± 0.001 to 1.102 ± 0.001 with the summary of the biofilm inhibition as shown in Figures 1 and 2. The effect of different concentrations ranging from 1 to 32 mg/ml of each of the extracts on the C. albicans biofilms established the reduction of biofilm by various extracts. Biofilm formed in the absence of the extracts was used as negative control. The biofilm inhibition potentials of the A. indica extract varied from 10.1 to 83.4% with the highest IC50 of 1.57 ± 0.01 mg/mL against C. albicans 4 (Figure 1, Table 7), while that of the C. roseus varied from 35.2 to 98.82% with the best IC50 of 1.29 ± 0.01 mg/mL against C. albicans 4 (Figure 2, Table 7). All the C. albicans strains showed reduced biofilm formation in the presence of different concentrations of the test extracts. The MRSA strain also showed reduced biofilm formation in the presence of different concentrations of the test extracts (Figure 3, Table 7). Biofilm formation was determined by crystal violet staining. Values are means of three independent experiments. P < 0.05 denotes between growth control and different concentrations of the extracts.
Figure 1.
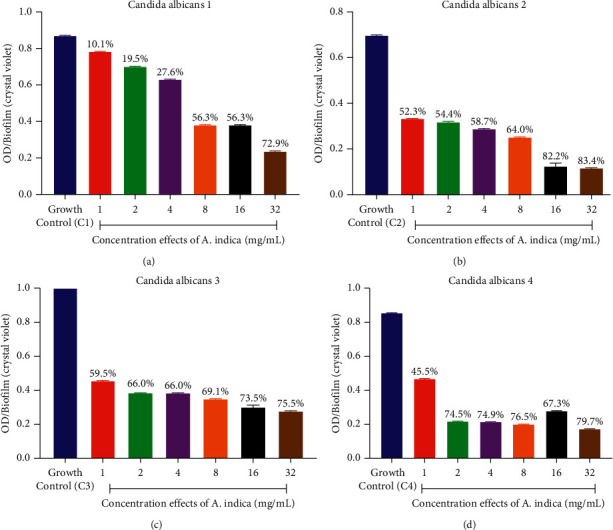
(a–d) Graphs showing the effect of different concentrations of ethanolic extracts of A. indica on the amount of biofilm formed (optical density (OD)) by fluconazole-resistant C. albicans strains.
Figure 2.
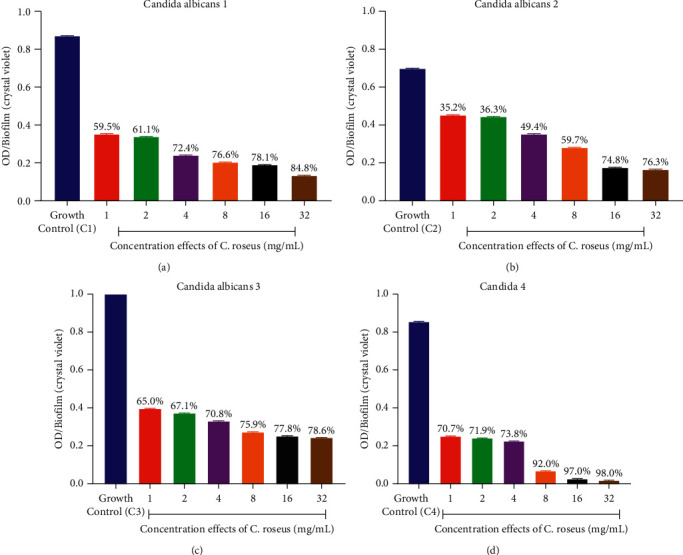
(a–d) Graphs showing the effect of different concentrations of ethanolic extracts of C. roseus on the amount of biofilm formed (optical density (OD)) by fluconazole-resistant C. albicans strains.
Table 7.
IC50 values of biofilm inhibition by ethanolic extracts of A. indica and C. roseus against fluconazole-resistant C. albicans strains and MRSA (NCTC12493).
| A. indica | C. roseus | ||
|---|---|---|---|
| Test organisms | IC50 values (mg/mL) | Test organisms | IC50 values (mg/mL) |
| CA1 | 8.87 ± 0.10 | CA1 | 1.64 ± 0.01 |
| CA2 | 2.39 ± 0.02 | CA2 | 3.81 ± 0.06 |
| CA3 | 1.69 ± 0.01 | CA3 | 1.50 ± 0.01 |
| CA4 | 1.57 ± 0.01 | CA4 | 1.29 ± 0.01 |
| MRSA (NCTC12493) | 1.002 ± 0.001 | MRSA | 1.73 ± 0.002 |
| Control | — | Control | — |
Each value is the average of three independent experiments ± SDs. CA1: Candida albicans 1, CA2: Candida albicans 2, CA3: Candida albicans 3, CA4: Candida albicans 4, MIC: minimum inhibitory concentration, and “—” no activity.
Figure 3.
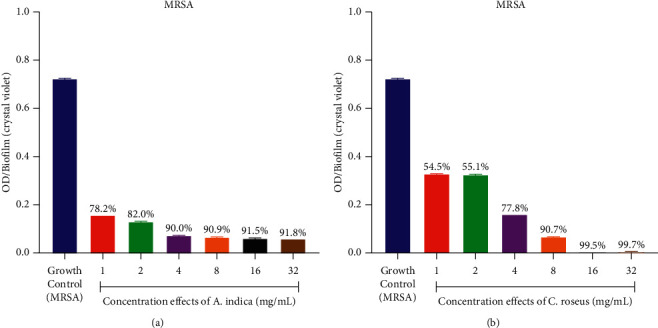
(a-b) Graphs showing the effect of different concentrations of ethanolic extracts of A. indica and C. roseus on the amount of biofilm formed (optical density (OD)) by MRSA (NCTC12493).
3.6. Time-Kill Kinetics Assay of Ethanolic Extracts of A. indica and C. roseus against Fluconazole-Resistant Strains of Candida albicans
Time-kill curves were performed for the resistant C. albicans strains using different concentrations of A. indica and C. roseus extracts with the MIC values ranging from 0.5 to 8 times. The results obtained for the time-kill curves are summarised in Figures 4 and 5 for each, respectively. The effects against the C. albicans strains were fungistatic (P < 0.05). Fungistatic activity has been defined as <3 log reduction in CFU/mL using time-kill.
Figure 4.
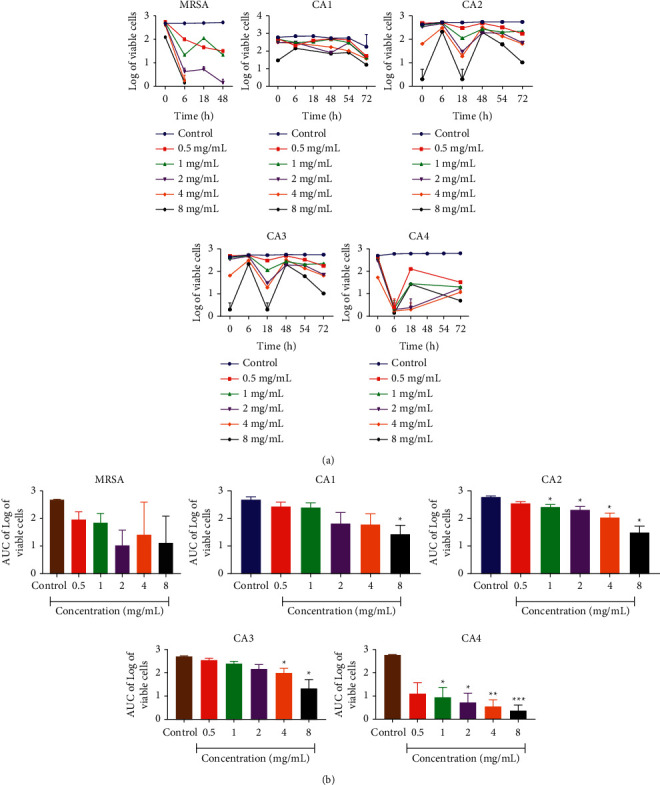
Time-kill kinetics of A. indica ethanolic extract against fluconazole-resistant C. albicans strains and MRSA (NCTC12493). (a) Time-kill kinetics curve and (b) AUC of time-kill kinetics. n = 5; values are mean ± SEM. ∗,∗∗p < 0.0001 (one-way ANOVA followed by Dunnett's post hoc test); AUC: area under the curve, CA1: C. albicans 1, CA2: C. albicans 2, CA3: C. albicans 3, and CA4: C. albicans 4.
Figure 5.
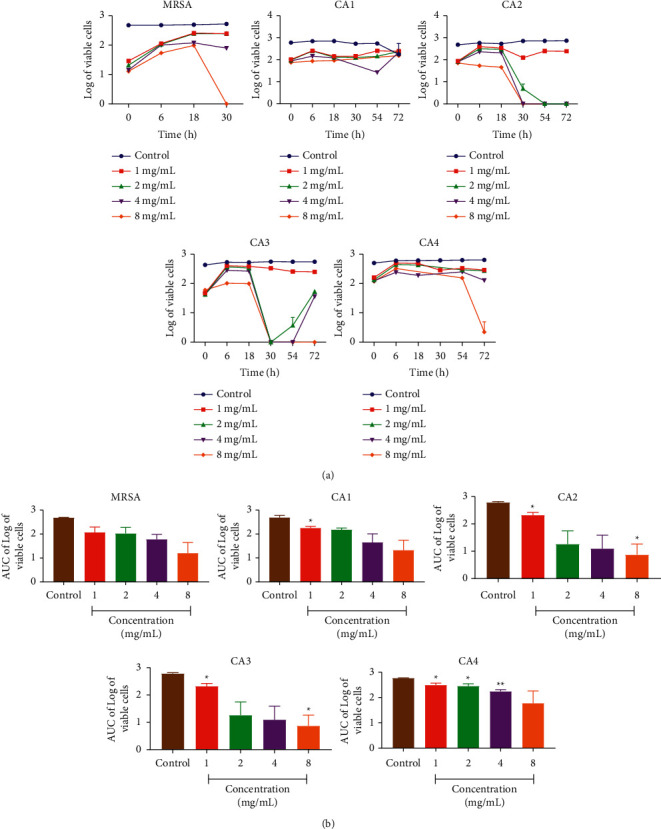
Time-kill kinetics of C. roseus ethanolic extract against fluconazole-resistant C. albicans strains and MRSA (NCTC12493). (a)Time-kill kinetics curve and (b) AUC of time-kill kinetics. n = 5; values are mean ± SEM. ∗,∗∗p < 0.0001 (one-way ANOVA followed by Dunnett's post hoc test); AUC: area under the curve, CA1: C. albicans 1, CA2: C. albicans 2, CA3: C. albicans 3, and CA4: C. albicans 4.
The present study allows us to establish that A. indica leaf and C. roseus flower extracts has a fungistatic effect against all the Candida albicans strains used.
4. Discussion
Antimicrobial agents have been used in contemporary medicine to help fight microbial infections [31]. However, the emergence and spread of antimicrobial resistance (AMR) threatens the effective control and management of various microbial infections worldwide [31]. The progress made in reducing mortality and morbidity as a result of early use of antibiotics based on empiric guidelines is under serious threat if steps are not taken to curb the menace of AMR [32]. Methicillin-resistant Staphylococcus aureus (MRSA) and Candida albicans are microbial organisms which cause several infectious diseases and have proven to be resistant to commonly used antimicrobial agents. Several mechanisms have been postulated to be responsible for microbial organisms' ability to resistant antimicrobial agents. One of such mechanisms is the formation of biofilm, which is a complex structure of microbiome with either different bacterial colonies or single type of cells, which tend to adhere to surfaces [33].
In fact, many research scientists are in the business of looking for novel compounds which are effective against several microbial organisms responsible for common infectious diseases and has little or no side effect. This study investigated the antimicrobial and antibiofilm effect of extracts of C. roseus and A. indica and their possible pharmacokinetic interactions with already known antimicrobial agents.
In the antimicrobial study, all the extracts significantly inhibited the growth of MRSA at the concentrations used. C. roseus has been reported by Shil et al. [34] to have antimicrobial property against S. aureus, confirming the findings of this study. The results on the antimicrobial activity of A. indica extract corroborate with studies done by Quelemes et al. [35] where they concluded that ethanolic leaf extract of A. indica inhibited the growth of MRSA. Several research works have shown that plants contain certain phytochemical constituents, which are responsible for their antimicrobial property. A review by Othman et al. [36] shows that alkaloids and polyphenols are largely responsible for plants' antimicrobial properties. These constituents were found in the two extracts used and hence may be responsible for the antimicrobial effect seen in this study.
The Candida albicans (CA) strains used in the antifungal experiment were clinical isolates from pregnant women. They were obtained from pregnant women because a work done by Masri et al. [37] revealed that pregnant women are a great source of CA and hence they are our choice for the source of the CA strains. All the extracts inhibited the growth of all the strains of CA used in this experiment indicating their antifungal property. This result was in tandem with some previous studies where they reported that various extracts of A. indica and C. roseus inhibited the growth of CA in their respective experiments [38, 39]. It is worthy of note that the standard drugs used, that is, fluconazole and voriconazole, which are triazole antifungal agents, could not inhibit the growth of all the CA strains used, confirming that the strains used were fluconazole-resistant.
One of the mechanisms used by microbial organisms to resist the effect of drugs is the formation of biofilm [40]. Bacteria in biofilm are surrounded by an extracellular matrix, which may physically prevent the penetration of antimicrobial agent through the cell wall of the microbial organism [40]. Ideally, antibiofilm property is an important trait that is exhibited by new antimicrobial agents. In this experiment, the ability of MRSA and CA to produce biofilms as a protective measure was established. However, simultaneous culturing of the microbial organisms and the various extracts showed a significant inhibition of the biofilm in all the strains used in this experiment indicating the antibiofilm property of both A. indica and C. roseus extracts. This corroborated with the earlier studies where they revealed that A. indica inhibited biofilm formation of Pseudomonas aeruginosa whereas C. roseus inhibited biofilm formation by P. aeruginosa and S. aureus, respectively [41, 42]. The ability of the extracts to inhibit biofilm formation could be attributed to the fact that their phytoconstituents could possibly destroy the structure of the microbial organism and also prevent the synthesis of peptidoglycan [43]. This result indicates that these extracts could be used in the management of microbial infections, which are resistant to conventional antimicrobial agents due to their ability to produce biofilms [14].
In the management of a number of infectious diseases, two or more drugs may however be employed. The therapeutic efficacy of the drug combination depends on the interactions of drugs combined. Synergistic combinations help to reduce emergence of resistant mutants and toxicity, exhibit more antimicrobial activity, and are more effective against mixed infections [44]. It is for this reason that an attempt was made in this study to ascertain potential pharmacodynamic interactions between the various concentration of the extracts and the standard drugs used against the experimental microbial organisms.
In the antifungal experiment, a combination of either A. indica or C. roseus extract and fluconazole or voriconazole showed several pharmacodynamic interactions on the different strains of the CA used. These interactions include antagonism, synergism, and additive and partial synergism. The difference in the herb drug interactions despite the fact that constant concentrations of both extracts and standard drugs used may be as a result of the difference in the strains of C. albicans used since they were obtained from different subjects. This interesting finding brings back the topic of pharmacogenomics where scientists are of the view that an individual's genetic make-up influences his or her response to drug therapy [45].
With regard to the herb drug interaction in the MRSA experiment, a combination of A. indica (AI) with either ampicillin or tetracycline showed a synergistic relationship, whereas antagonism was revealed in the combination of AI and streptomycin. This result however was opposite to the findings made by Ngwu et al. [46] where there was rather an antagonism observed with AI and tetracycline and synergism observed with AI and streptomycin. This observation may be due to the difference in the source of AI used since it is believed that regional and climate difference may affect active constituents found in medicinal plants [36]. With regard to C. roseus (CR) and ampicillin combination, there was an antagonistic interaction whilst there was partial synergy in the tetracycline and CR combination. This result was in contrast with that recorded by Shil et al. [34] where CR had a synergistic interaction with ampicillin. The difference in results may be attributed to the organism used; whilst they used multiple drug-resistant S. aureus strain, in this experiment MRSA was used. Again, different climate conditions could also affect the phytoconstituents in the CR used for the two studies.
Time-kill kinetics assay is used to study the activity of an antimicrobial agent against microorganisms and to further categorize them into either bactericidal and or fungicidal or bacteriostatic and or fungistatic. In this experiment, AI and CR were bacteriostatic and fungistatic, respectively.
It could be concluded that A. indica and C. roseus extracts possess interesting antimicrobial and antibiofilm activity and have the potential to have various pharmacodynamic interactions with standard antimicrobial agents.
5. Conclusion
The discovery of new and effective natural bioactive compounds with high antifungal and anti-MRSA activities, specifically biofilm-forming cells, will signify a substantial impact on the treatment and management of C. albicans infections as well as other related bacterial infections. Our results therefore indicated that the extracts of A. indica leaf and C. roseus flower alone or in combination with the test antifungals could provide a promising means of the management of vulvovaginal candidiasis caused by drug-resistant strains as well as when in combination with the selected antibacterial agents against recurrent infections caused by the MRSA. However, additional researches are required to identify the antimicrobial activity of A. indica leaf and C. roseus flower and its bioactive elements against C. albicans and non-C. albicans species implicated in different clinical infections, not only vulvovaginal candidiasis as well as other resistant bacterial strains aside MRSA.
Data Availability
All data are included in the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and Global Health . 2015;109(7):309–318. doi: 10.1179/2047773215y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Who. WHO Report on Antimicrobial Resistance-A Global Epidemic . Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 3.Ventola C. L. The antibiotic resistance crisis: part 1: causes and threats,” P & T. A Peer-Reviewed Journal for Formulary Management . 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 4.Clontz L. Biofilm inhibition: the use of a marine alkaloid derivative in the prevention of clinically-relevant biofilms. Journal of Microbiology & Experimentation . 2018;6(5):206–214. doi: 10.15406/jmen.2018.06.00216. [DOI] [Google Scholar]
- 5.Raygada J. L., Levine D. P. Methicillin-resistant Staphylococcus aureus: a growing risk in the hospital and in the community. American Health & Drug Benefits . 2009;2(2):86–95. [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S. S., Hinrichsen V. L., Datta R., et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One . 2011;6 doi: 10.1371/journal.pone.0024340.e24340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainsa J. Stuart B. Levy: the antibiotic paradox. How the misuse of antibiotics destroys their curative powers, 2nd edn. International Microbiology . (2nd) 2002;5(3):155–156. doi: 10.1007/s10123-002-0082-z. [DOI] [Google Scholar]
- 8.Chen C. H., Gau V., Zhang D. D., Liao J. C., Wang F.-Y., Wong P. K. Statistical metamodeling for revealing synergistic antimicrobial interactions. PLoS One . 2010;5(11) doi: 10.1371/journal.pone.0015472.e15472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zida A., Bamba S., Yacouba A., Ouedraogo-Traore R., Guiguemdé R. T. Anti-Candida albicans natural products, sources of new antifungal drugs: a review. Journal De Mycologie Médicale . 2017;27(1):1–19. doi: 10.1016/j.mycmed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Jabra-Rizk M. A., Falkler W. A., Meiller T. F. Fungal biofilms and drug resistance. Emerging Infectious Diseases . 2004;10(1):p. 14. doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage G., Martínez J. P., López-Ribot J. L. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Research . 2006;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 12.Aghajanyan A., Ginovyan M., Trchounian A. Herbs extracts in the treatment and prevention of experimental metabolic disorders: synergistic hypoglycemic activity of ethanol extracts of Hypericum alpestre and Rumex obtusifolius. Multidisciplinary Digital Publishing Institute Proceedings . 2019;11 doi: 10.3390/proceedings2019011002. [DOI] [Google Scholar]
- 13.Figueiredo C., Garcia‐Gonzalez M. A., Machado J. C. Molecular pathogenesis of gastric cancer. Helicobacter . 2013;18:28–33. doi: 10.1111/hel.12083. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin P. R., Reeves A. Z., Powell K. R., et al. Monocarbonyl analogs of curcumin inhibit growth of antibiotic sensitive and resistant strains of Mycobacterium tuberculosis. European Journal of Medicinal Chemistry . 2015;92:693–699. doi: 10.1016/j.ejmech.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farnsworth N. R., Akerele O., Bingel A. S., Soejarto D. D., Guo Z. Medicinal plants in therapy. Bulletin of the World Health Organization . 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 16.Iranmai M. K., Sha S. B. U., Udharshan R. D. S., Ahendra C. B. M. K., Ibrahim M. Evaluation of total phenolic contents and antiulcerogenic activity of root bark of Azadirachta indica. Global Journal of Medical Research . 2012;12(6):23–29. [Google Scholar]
- 17.Das S., Sharangi A. B. Madagascar periwinkle (Catharanthus roseus L.): diverse medicinal and therapeutic benefits to humankind. Journal of Pharmacognosy and Phytochemistry . 2017;6(5):1695–1701. [Google Scholar]
- 18.Patil P. J., Ghosh J. S. Antimicrobial activity of Catharanthus roseus–a detailed study. British Journal of Pharmacology and Toxicology . 2010;1(1):40–44. [Google Scholar]
- 19.Verma A. K., Singh R. R. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian Journal of Pharmaceutical Sciences . 2010;72(5):p. 655. doi: 10.4103/0250-474x.78541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassyouni R. H., Wegdan A. A., Abdelmoneim A., Said W., AboElnaga F. Phospholipase and aspartyl proteinase activities of candida species causing vulvovaginal candidiasis in patients with type 2 diabetes mellitus. Journal of Microbiology and Biotechnology . 2015;25(10):1734–1741. doi: 10.4014/jmb.1504.04009. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller M. A., Diekema D. J., Sheehan D. J. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clinical Microbiology Reviews . 2006;19(2):435–447. doi: 10.1128/cmr.19.2.435-447.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer J. J., Dilika F. Antibacterial activity of Helichrysum pedunculatum used in circumcision rites. Journal of Ethnopharmacology . 1996;53(1):51–54. doi: 10.1016/0378-8741(96)01411-0. [DOI] [PubMed] [Google Scholar]
- 23.Visweswari G., Christopher R., Rajendra W. Phytochemical screening of active secondary metabolites present in Withania somnifera root: role in traditional medicine. International Journal of Pharmaceutical Sciences and Research . 2013;4(7):p. 2770. [Google Scholar]
- 24.Neglo D., Tettey C. O., Essuman E. K., et al. Evaluation of the modulatory effect of annona muricata extracts on the activity of some selected antibiotics against biofilm-forming MRSA. Evidence-Based Complementary and Alternative Medicine . 2021;2021:9. doi: 10.1155/2021/9342110.9342110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eloff J. N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica . 1998;64(8):711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 26.Khodavandi A., Alizadeh F., Aala F., Sekawi Z., Chong P. P. In vitro investigation of antifungal activity of allicin alone and in combination with azoles against Candida species. Mycopathologia . 2010;169(4):287–295. doi: 10.1007/s11046-009-9251-3. [DOI] [PubMed] [Google Scholar]
- 27.Dickson R. A., Houghton P. J., Hylands P. J., Gibbons S. Antimicrobial, resistance‐modifying effects, antioxidant and free radical scavenging activities of Mezoneuron benthamianum Baill., Securinega virosa Roxb. &Wlld. and Microglossa pyrifolia Lam. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives . 2006;20(1):41–45. doi: 10.1002/ptr.1799. [DOI] [PubMed] [Google Scholar]
- 28.Haque F., Alfatah M., Ganesan K., Bhattacharyya M. S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Scientific Reports . 2016;6(1):1–11. doi: 10.1038/srep23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alshami I., Alharbi A. E. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pacific Journal of Tropical Biomedicine . 2014;4(2):104–108. doi: 10.1016/s2221-1691(14)60217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appiah T., Boakye Y. D., Agyare C. Antimicrobial activities and time-kill kinetics of extracts of selected Ghanaian mushrooms. Evidence-Based Complementary and Alternative Medicine . 2017;2017:15. doi: 10.1155/2017/4534350.4534350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aslam B., Wang W., Arshad M. I., et al. Antibiotic resistance: a rundown of a global crisis. Infection and Drug Resistance . 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gera T., Shah D., Garner P., Richardson M., Sachdev H. S. Integrated management of childhood illness (IMCI) strategy for children under five. Cochrane Database of Systematic Reviews . 2016;6(6) doi: 10.1002/14651858.CD010123.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma D., Misba L., Khan A. U. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrobial Resistance and Infection Control . 2019;8(1):p. 76. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shil A., Mukherjee S., Bishayi B., Bhakta M. S. A comparison of antibacterial effects of Catharanthus roseus and Camellia sinensis (Black Tea) and their synergistic effect along with antibiotic against multiple antibiotic resistant strains of Staphylococcus aureus. Journal of Herbs, Spices, & Medicinal Plants . 2021;27(2):135–148. doi: 10.1080/10496475.2020.1815921. [DOI] [Google Scholar]
- 35.Quelemes P. V., Perfeito M. L. G., Guimarães M. A., et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. Journal of Ethnopharmacology . 2015;175:287–294. doi: 10.1016/j.jep.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Othman L., Sleiman A., Abdel-Massih R. M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Frontiers in Microbiology . 2019;10 doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masri S. N., Noor S. M., Mat Nor L. A., Osman M., Rahman M. M. Candida isolates from pregnant women and their antifungal susceptibility in a Malaysian tertiary-care hospital. Pakistan Journal of Medical Sciences . 2015;31(3):658–661. doi: 10.12669/pjms.313.7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balaabirami S., Patharajan S. In vitro antimicrobial and antifungal activity of Catharnthus roseus leaves extract against important pathogenic organisms. International Journal of Pharmacy and Pharmaceutical Sciences . 2012;4(SUPPL.3):487–490. [Google Scholar]
- 39.Mahmoud D. A., Hassanein N. M., Youssef K. A., Abou Zeid M. A. Das bau-SOLL im tunnelbau wo liegen die missverständnisse wo liegen die größten irrtümer. Brazilian Journal of Microbiology: Publication of the Brazilian Society for Microbiology . 2011;42(3):1007–1016. doi: 10.1590/s1517-83822011000300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel R. Biofilms and antimicrobial resistance. Clinical Orthopaedics and Related Research . 2005;437:41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- 41.Alavi M., Karimi N. Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag-TiO2, TiO2-Ag, Ag-Cu and Cu-Ag nanocomposites against multi-drug-resistant bacteria. Artificial Cells, Nanomedicine, and Biotechnology . 2018;46(sup3):S399–S413. doi: 10.1080/21691401.2018.1496923. [DOI] [PubMed] [Google Scholar]
- 42.Harjai K., Bala A., Gupta R. K., Sharma R. Leaf extract of Azadirachta indica (neem): a potential antibiofilm agent for Pseudomonas aeruginosa. Pathogens and Disease . 2013;69(1):62–65. doi: 10.1111/2049-632X.12050. [DOI] [PubMed] [Google Scholar]
- 43.Cox S. D., Mann C. M., Markham J. L., Bell H. C., Gustafson J. E., Wyllie S. G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) Journal of Applied Microbiology . 2000;88(1):170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 44.Bhardwaj M., Singh B. R., Sinha D. K., et al. Potential of herbal drug and antibiotic combination therapy: a new approach to treat multidrug resistant bacteria. Pharmaceutica Analytica Acta . 2016;7(11) doi: 10.4172/2153-2435.1000523. [DOI] [Google Scholar]
- 45.Awaisu A., Elewa H. Pharmacogenomics in pharmacy practice: current perspectives. Integrated Pharmacy Research and Practice . 2019;8:97–104. doi: 10.2147/IPRP.S180154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngwu M. I., Adikwu M. U., Ibezim E. C., Odimegwu D. C., Ngwu G. I., Esimone C. O. Susceptibility pattern of a clinical isolate of Staphylococcus aureus to the combined activity of a herbal preparation of Azadirachta indica and some antibiotics. International Journal of Health Research . 2009;2(3):279–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript.


