Figure 1.
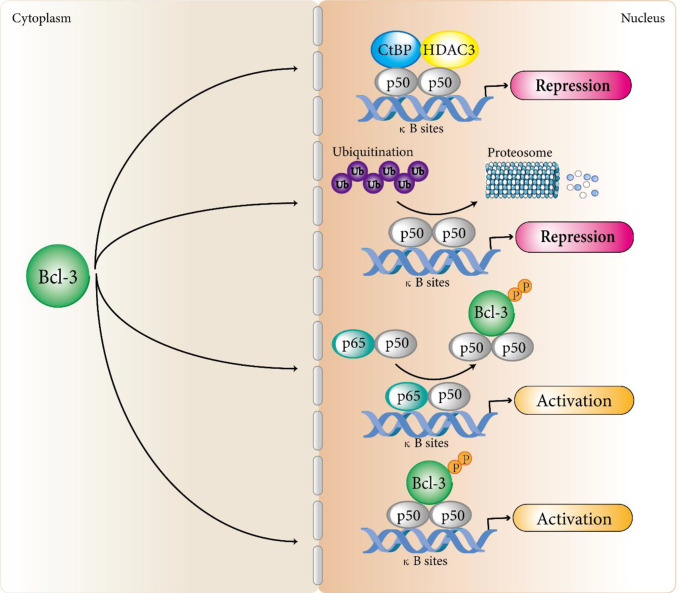
Bcl-3 regulates atypical NF-κB signaling pathways. Bcl-3 acts as a regulator of the atypical NF-κB pathway by binding to processed p50 and p52 homodimers to repress or activate a subset of NF-κB regulated genes. In terms of transcriptional inhibition, Bcl-3 delays the turnover of DNA binding inhibitory p50 homodimer by inhibiting the ubiquitination of p50 homodimer and subsequent proteasome hydrolysis, so as to produce a stable DNA binding complex and inhibit transcription. In addition, the recruitment of co-repressors such as CtBP and HDAC3 may be another mechanism by which Bcl-3 inhibits transcription of NF-κB target genes. In terms of transcriptional activation, Bcl-3 removes repressive p50 homodimers from NF-κB sites, allowing NF-κB heterodimers associated with classical signaling (p65/p50) to activate transcription at these sites. Bcl-3 can also directly transactivate NF-κB-dependent transcription with N-terminal and C-terminal domains by binding to p50 and p52 homodimers. Although Bcl-3 can directly interact with p52 homodimers, the mechanism of Bcl-3 regulating p52 homodimer activation still remains unclear and we speculate that it follows a similar mechanism to that of the p50 homodimers.