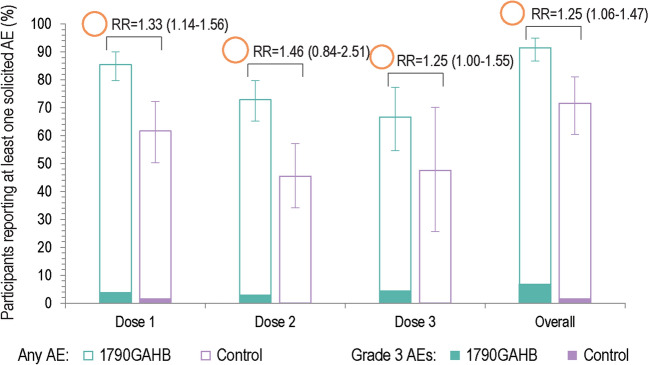
Fig. 5.
Summary of solicited adverse events reported within 7 days after each dose and overall (solicited safety set). AE adverse event, RR risk ratio. Solicited AEs collected in the studies were pain, erythema, induration, swelling, facial edema, nasal pain, rhinorrhea (local), and arthralgia, chills, fatigue, headache, malaise, myalgia, and fever (systemic). The area of the orange circles is proportional to the values of risk ratios/between-group ratios