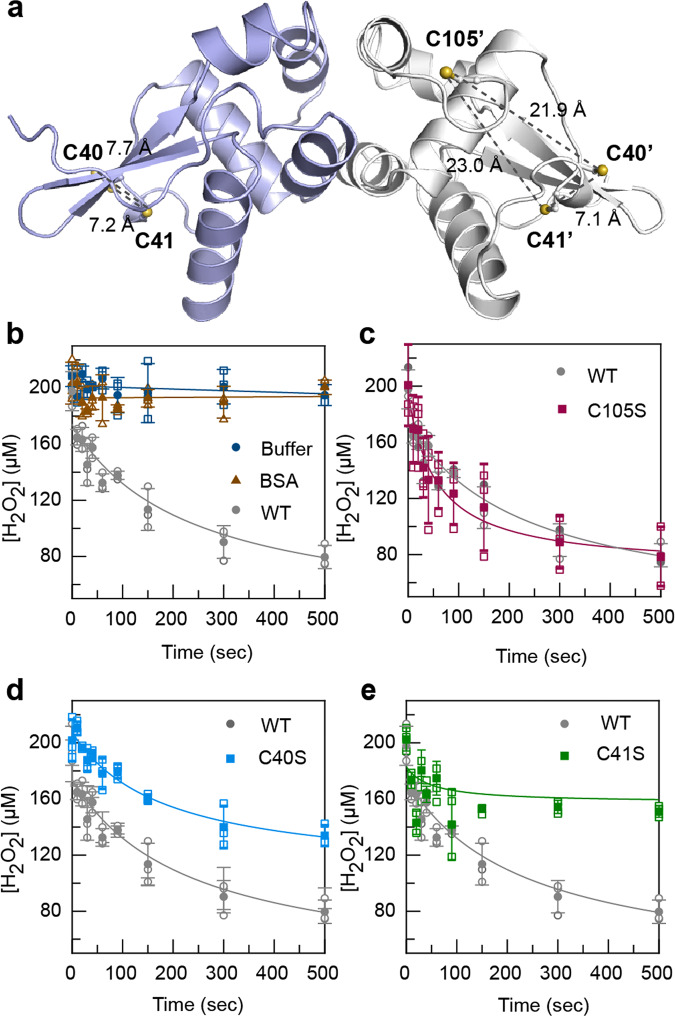
Fig. 3. Cys40 and Cys41 form an intramolecular disulfide bond in response to H2O2 stress.
a The positions of the Cys residues in the reduced structure of RexT. The two sulfur atoms of Cys40 and Cys41 are located ~7 Å apart in both monomeric units of RexT. The sulfur atoms of Cys40 and Cys41 are located 21.9 Å and 23.0 Å away from the sulfur atom of Cys105, the last modeled residue in the crystal structure, respectively (shown for chain B of the structure). There are two measurements shown in chain A since the Cys41 residue sidechain shows two orientations of the sidechain. b Using the FOX assay, wild-type RexT was shown to consume H2O2 over time. Two control reactions are also included in this panel that shows H2O2 is not consumed in the absence of RexT or in the presence of the protein bovine serum albumin (BSA). c The C105S RexT variant consumes H2O2 similarly to wild-type RexT, suggesting it is not involved in mediating the oxidative stress response. d In contrast, the C40S variant of RexT shows a decreased ability to consume H2O2 relative to wild-type RexT. e As observed for the C40S variant, the C41S RexT variant is also impaired in its ability to consume H2O2, albeit to a greater extent. In b–e, data was measured using n = 3 independent experiments and is presented with the individual measurements (open shapes) and as the mean value of these measurements ± SD (closed shapes). Source data are provided as a Source Data file.