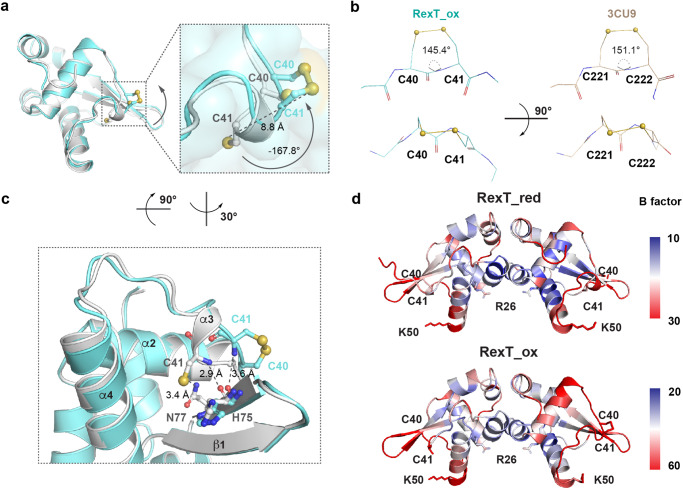
Fig. 4. Disulfide bond formation in RexT causes a conformational change and loss of interactions.
a An overlay of chain B from the structures of the reduced (light gray) and oxidized (teal) states of RexT. A box is included to highlight the Cys residues involved in disulfide bond formation. The formation of the disulfide bond requires Cys41 to undergo a large conformational change. b Comparison of the eight-membered vicinal disulfide bond geometry in RexT and 1, 5-alpha-L-arabinanase37 (PDB ID: 3CU9). The ω angles of both RexT and 1, 5-alpha-L-arabinanase show a large deviation from the ideal values. c Upon disulfide bond formation in RexT, the movement of Cys41 causes disruptions in the interactions between the thiol-group and the sidechain of Asn77 as well as interactions between the Cys41 backbone amide and the backbone of His75. Both Asn77 and His75 are found in the wing portion of the wHTH motif involved in DNA binding. d The B factors of the reduced (top panel, RexT_red) and oxidized (bottom panel, RexT_ox) states of RexT are illustrated in a continuum from blue, white to red. Blue corresponds to a lower B factor and red represents a higher B factor. The reduced structure is colored on a scale from 10 to 30, whereas the oxidized structure is colored on a scale from 20 to 60.