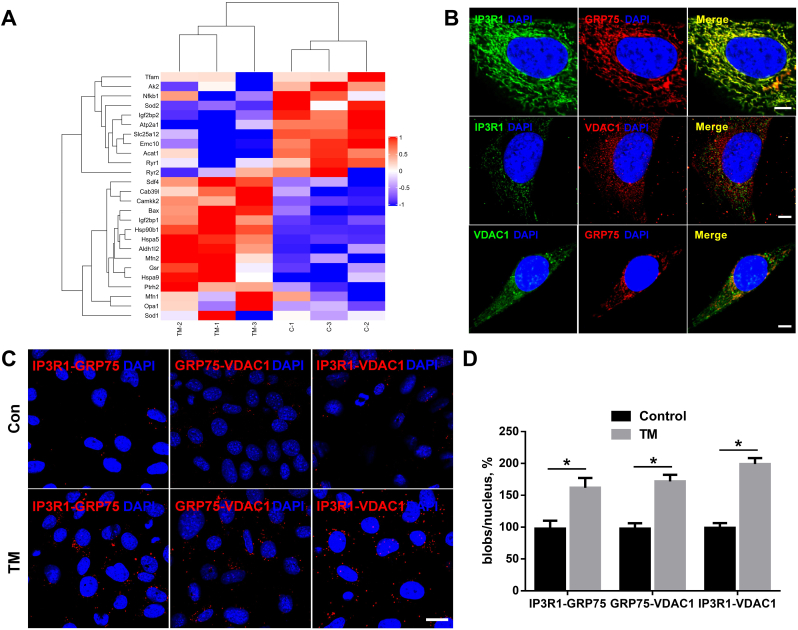
Fig. 2.
The IP3R1–GRP75–VDAC1 complex plays an important role between the ER and the mitochondria in the heart.
A, HL-1 cells were cultured with tunicamycin (TM) for 24 h. Mass spectrometry identified GRP75 as a strong interacting protein. B, Conventional immunofluorescence confirming the colocalization of IP3R1, GRP75, and VDAC1 in HL-1 cells. Scale bar = 5 μm. C, D, In situ proximity ligation assay (PLA) monitoring of perturbation of ER–mitochondria interactions linked to genetic modulation of IP3R1–GRP75–VDAC1 complex proteins. Shown are representative PLA images (C) and quantitative analysis (D) of IP3R1–GRP75, GRP75–VDAC1, and IP3R1–VDAC1 interactions in HL-1 cells cultured with control or with TM for 24 h. Scale bar = 20 μm. Values are the mean ± SEM, n = 4 independent experiments, *P < 0.05, two-tailed Student's t-test.