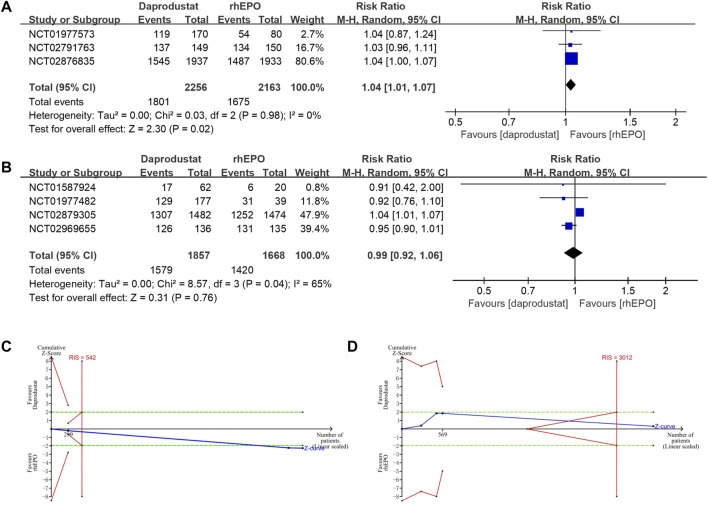
FIGURE 4.
The adverse events of daprodustat. (A) Forest plot of the adverse events of daprodustat and rhEPO in NDD-CKD patients. Notes: RR = 1.04, 95% CI = 1.01, 1.07, p = 0.02, I2 = 0%. (B) Forest plot of the adverse events of daprodustat and rhEPO in DD-CKD patients. Notes: RR = 0.99, 95% CI = 0.92, 1.06, p = 0.76, I2 = 65%. (C) Random effects model of the TSA of adverse events of daprodustat and rhEPO in NDD-CKD patients. A diversity-adjusted information size of 542 participants was calculated based on an adverse event rate of 77.4% in the rhEPO group and a RR reduction of 15%, with α = 5% (two-sided), β = 15%, and I2 = 0%. The solid blue line represents the cumulative Z-curve, which crossed the futility boundary (solid red line). (D) Random effects model of the TSA of adverse events of daprodustat and rhEPO in DD-CKD patients. A diversity-adjusted information size of 3012 participants was calculated based on an adverse event rate of 85.1% in the rhEPO group and a RR reduction of 15%, with α = 5% (two-sided), β = 15%, and I2 = 65%. The solid blue line represents the cumulative Z-curve, which crossed the futility boundary (solid red line).