Abstract
The neurotrophin Nerve Growth Factor (NGF) holds a great potential as a therapeutic candidate for the treatment of neurological diseases. However, its safe and effective delivery to the brain is limited by the fact that NGF needs to be selectively targeted to the brain, to avoid severe side effects such as pain and to bypass the blood brain barrier. In this perspective, we will summarize the different approaches that have been used, or are currently applied, to deliver NGF to the brain, during preclinical and clinical trials to develop NGF as a therapeutic drug for Alzheimer’s disease. We will focus on the intranasal delivery of NGF, an approach that is used to deliver proteins to the brain in a non-invasive, safe, and effective manner minimizing systemic exposure. We will also describe the main experimental facts related to the effective intranasal delivery of a mutant form of NGF [painless NGF, human nerve growth factor painless (hNGFp)] in mouse models of Alzheimer’s disease and compare it to other ways to deliver NGF to the brain. We will also report new data on the application of intranasal delivery of hNGFp in Down Syndrome mouse model. These new data extend the therapeutic potential of hNGFp for the treatment of the dementia that is progressively associated to Down Syndrome. In conclusion, we will show how this approach can be a promising strategy and a potential solution for other unmet medical needs of safely and effectively delivering this neuroprotective neurotrophin to the brain.
Keywords: nerve growth factor, down syndrome, intranasal, neurogenesis, inflammation, microglia, astrocytes
Introduction
The neurotrophin Nerve Growth Factor (NGF) (Levi-Montalcini, 1952) has been suggested to play a neuroprotective factor in several neurological diseases and has been a matter of numerous basic and clinical research studies.
Nerve growth factor is produced from a gene located on chromosome 1 (Francke et al., 1983) as a precursor proNGF which exists in two distinct isoforms of 27 and 35 kDa (Edwards et al., 1988). ProNGF is then cleaved to mature NGF by a group of enzymes including furin and metalloproteases (Cuello et al., 2019). Recently, it has been found that both proNGF and mature NGF can trigger biological responses (Lee et al., 2001), most often with an opposite sign. First, it has been demonstrated that proNGF is the major species of this neurotrophin both in the Central Nervous Systems (Fahnestock and Shekari, 2019). Secondly, proNGF can bind p75NTR, the receptor common to all neurotrophins. But it can also bind to the tropomyosin-related kinase receptor TrkA, although with a lower affinity than mature NGF (Fahnestock et al., 2004). Thus, a subtle balance between the amount of proNGF, mature NGF and their receptors can lead to either a neuroprotective or a pro-neurodegenerative outcome (see below).
Nerve Growth Factor in Alzheimer’s Disease and its Limits to Clinical Application
Alzheimer’s disease (AD) neuropathology is distinguished by deposits of misfolded proteins, mainly consisting of hyperphosphorylated tau and β-amyloid (Aβ) (Selkoe, 2001). Another prominent feature of the neurodegenerative process characterizing AD is the occurrence of cholinergic deficit (Whitehouse et al., 1982), which put the theoretical basis of the pharmacological therapy available for AD patients (use of cholinesterase inhibitors) (Giacobini, 2006). Basal forebrain cholinergic neurons (BFCNs) were identified as the most significant NGF-sensitive population inside the CNS. These neurons express both NGF receptors TrkA and p75NTR (Hefti, 1986; Holtzman et al., 1992), are able to retrogradely transport NGF from their cortical projections up to their cell bodies (Seiler and Schwab, 1984) and respond to administration of exogenous NGF, in terms of increase of cholinergic phenotypical markers (Gnahn et al., 1983; Mobley et al., 1986). Most importantly, NGF is able to prevent BFCN death or atrophy, following axotomy (Hefti, 1986; Williams et al., 1986; Kromer, 1987) or linked to aging (Fischer et al., 1987). In AD, a selective decrease in the expression of TrkA, and not p75NTR, occurs in BFNCs and hippocampus and it correlates with the severity of the disease (Mufson et al., 2019). The distinctive cholinergic deficit in AD, together with the BFCN being NGF target neurons, has led to propose the use of NGF as a treatment for AD (Tuszynski et al., 2005; Mitra et al., 2019).
Work with the anti-NGF AD11 mouse model (Ruberti et al., 2000), in which the expression of antibodies against mature NGF in the adult brain causes a progressive neurodegeneration which is similar to that observed in AD brains, provided the first demonstration that deficits in NGF signaling may lead to a Alzheimer-like neurodegeneration (Capsoni et al., 2011), which is broader than a pure cholinergic deficit. This comprehensive neurodegeneration phenotype suggested that other cells in the brain, in addition to BFCNs, might respond to NGF deficits and, conversely, might represent targets for NGF therapeutic actions. Indeed, triggered by this neurodegeneration picture, we found that microglia are NGF target cells and respond to NGF by activating a potent and broad neuroprotective and anti-inflammatory action (Rizzi et al., 2018).
Deficits in NGF processing or transport could be causally linked to the onset of AD neurodegeneration. Whilst in the AD11 model the NGF deficit is determined by interference with an anti-NGF antibody expressed in the brain, different pathological mechanisms could result in a reduced NGF bioactivity. Thus, a reduced NGF bioactivity might result either by a defect in NGF retrograde transport system (Mufson et al., 1995) or by an unbalance of proNGF vs. mature NGF signaling (Fahnestock et al., 2001; Podlesniy et al., 2006). Indeed, experimentally increasing proNGF in transgenic mice also induces a progressive neurodegeneration, despite concomitant higher-than-normal mature NGF levels (Tiveron et al., 2013; Fasulo et al., 2017).
We can therefore formulate an NGF hypothesis for AD neurodegeneration, whereby a common link behind AD neurodegeneration is a failure or an insufficient NGF signaling, leading to inadequate neurotrophic support (Capsoni and Cattaneo, 2006; Cattaneo et al., 2008; Cattaneo and Calissano, 2012). The failure or unbalance of NGF support could be due to different causes in the overall cascade(s) of events involving NGF bioactivity: (1) decreased NGF synthesis, (2) unbalanced or altered processing, (3) alterations in receptor expression and/or activity or expression ratios, and (4) altered retrograde transport. These events would be “located” upstream of the “amyloid cascade,” which is the central core of AD neurodegeneration, as currently described (Selkoe, 2000), and would be part of a negative feedback loop that involves several steps (e.g., links between APP, tau, and axonal transport). On the other hand, the intrahippocampal injection of Aβ oligomers in naïve rats is sufficient to induce a proNGF/NGF unbalance (Bruno et al., 2009).
Thus, an initial deficit in NGF signaling or processing or a reduction of TrkA receptors will result in a feed-forward pathological cycle leading to increased accumulation of Aβ and propagation of proNGF/NGF homeostasis deficits (Cattaneo and Capsoni, 2019).
Within this theoretical frame, any therapy aimed at re-establishing the correct balance between ligands (and receptors) of the NGF pathway appears to have a clear rationale. The most direct therapeutic approach along these lines would be, therefore, to exploit NGF itself. However, the viable clinical application of NGF requires providing a solution to major obstacles, namely finding a more effective NGF delivery to the CNS and limiting adverse effects deriving from undesired NGF actions, most notably, pain.
Past and Ongoing Nerve Growth Factor Clinical Trials in Alzheimer’s Disease
One approach to overcome the limits of NGF administration might be the use of small molecules that could cross the blood brain barrier and mimic NGF action, improving survival of target cells [Pediaditakis et al. (2016a,b) and reviewed in Gascon et al. (2021)]. Currently one of these small-molecule NGF mimetics, the P75NTR binding molecule LM11A-31, is under evaluation in clinical trials in Alzheimer’s disease (Yang et al., 2008, 2020). However, this approach might represent limitations due to a more restricted pharmacological profile with respect to that of the NGF protein.
To achieve a therapeutic concentration of NGF in the brain, while also avoiding systemic exposure, a first clinical has been performed in which an intracerebroventricular infusion was performed in three patients (Eriksdotter Jonhagen et al., 1998). Despite an increase in nicotinic receptor expression and an amelioration in cognitive function, the trial had to be stopped due to the onset of unbearable back pain linked to the diffusion of NGF in the CSF irrorating the spinal cord. For this reason, subsequent clinical trials were performed using cells engineered to secrete NGF or adenoviruses carrying the sequence encoding for NGF, stereotaxically implanted by neurosurgery close to the basal forebrain. In 2005 a clinical trial targeting the BFCNs was performed in 8 patients in which autologous fibroblasts were engineered to produce NGF. Using this approach, slowing down of the cognitive decline, associated with an amelioration of cortical glucose uptake, was found (Tuszynski et al., 2005, 2015). Lately, an NGF-encoding adeno-associated viral vector also injected in the basal forebrain has been used (Rafii et al., 2014) but the treatment did not lead to clinical efficacy, most likely because of the failure to accurately engage the target cells (Rafii et al., 2018).
More recently, clinical trials using the encapsulated cell biodelivery (ECB) have been started. The ECB cells engineered to secrete NGF are located at the tip of a catheter formed by a semipermeable membrane to allow the exchange of NGF and nutrients in the extracellular fluid (Lindvall and Wahlberg, 2008). These catheters have been implanted in the basal forebrain of AD patients and allow to achieve an increase in choline acetyltransferase activity and glucose content in the brain, and amelioration in memory tests (Mitra et al., 2019). However, despite the encouraging results, the trials have been slowed down because of the variability of results due to degeneration of the engineered cells (Mitra et al., 2019).
The Advantage of Intranasal Delivery vs. Ocular Delivery
To bypass the blood brain barrier, intranasal delivery is an alternative solution that has been proposed for several proteins (Dhuria et al., 2010; Malerba et al., 2011). As far as NGF is concerned, in 1997 Frey’s group used radioactive labeled NGF to demonstrate that the intranasal delivery allows to obtain NGF in therapeutic concentrations in several regions of rat brain (Frey et al., 1997). Several hypotheses have been formulated concerning the pathways through which the protein can reach the brain. These include nerves (olfactory and trigeminal) connecting the nasal passages to the brain, vasculature, cerebrospinal fluid (CSF) and lymphatic system [reviewed in Dhuria et al. (2010) and Malerba et al. (2011)]. Our laboratory first applied this technique in anti- NGF AD11 mice, and we showed that intranasally delivered NGF could reduce memory deficits and the accumulation of Aβ deposits, hyperphosphorylated tau and cholinergic deficiency (Capsoni et al., 2002; De Rosa et al., 2005). In a subsequent study, intranasal delivery of NGF was compared to the administration of NGF eye-drops. It was found that the ocular delivery of NGF was less efficient than nasal delivery in rescuing tau-related neurodegeneration in AD11 mice, since a ten times higher dose than the one used for intranasal delivery was necessary to obtain the same effect (Capsoni et al., 2009).
Microglia as a New Target for the Actions of Intranasal Painless Nerve Growth Factor (Human Nerve Growth Factor Painless)
Intranasal delivery allows not only to reach brain regions, but it also reduces the possibility to have a systemic leakage of the protein in blood circulation, thus reducing the possibility to trigger side effects such as pain. To increase the therapeutic index and to reduce the possibility to trigger nociceptor sensitization, a mutation in the human NGF gene, inspired by a rare human disease, the Hereditary Sensory and Autonomic Neuropathy type V (HSAN V), was introduced. HSAN V patients carry a mutation from arginine 100 to tryptophan (R100W) and suffer of pain insensitivity without having cognitive deficits (Einarsdottir et al., 2004). After screening different amino acid substitutions, we selected the mutation R100E because of (i) its similarity to the R100W mutation in selectively altering TrkA signaling, (ii) in abolishing the binding to p75NTR receptor and because of (iii) a more efficient production in Escherichia coli (Covaceuszach et al., 2010). In addition to the R100E mutation, a second one (P61S) was introduced to make the protein detectable against the endogenous human NGF (Covaceuszach et al., 2009). The mutant NGFP61SR100E [painless NGF or human nerve growth factor painless (hNGFp)] was shown to have the same neurotrophic potency as wildtype NGF, in several bioassays, while showing a greatly reduced pain sensitization potency, in a number of pain assays, with respect to wild type NGF (Malerba et al., 2015). From the pharmacological point of view, hNGFp is a TrkA-biased agonist, with a greatly reduced ability to bind and activate p75NTR (Cattaneo and Capsoni, 2019).
In a first study, hNGFp was used to treat AD11 and APPxPS1 mice (Capsoni et al., 2012). We showed that the intranasal delivery was able to improve memory in both transgenic models, as assessed by novel object recognition and in Morris water maze tests. Moreover, in both mouse models Aβ deposition was lowered in both transgenic mice. In AD11 mice, also tau hyperphosphorylation and cholinergic deficit were decreased.
A second paper in which the treatment was performed in 5xFAD mice allowed us to uncover the neuroprotective mechanisms through which hNGFp acts to reduce the neurodegeneration and to compare the effectiveness of intranasal delivery vs. a local delivery to cholinergic neurons, mimicking the approach used in clinical trials (Capsoni et al., 2017). First, we demonstrated that intranasal hNGFp can be detected at 6 and 24 h after the administration in the hippocampus and cerebral cortex, respectively, two areas highly affected by the neurodegeneration. We found that the local delivery of hNGFp to cholinergic neurons of the nucleus basalis was not decreasing the number of plaques in 5xFAD mice, despite the sprouting of cholinergic fibers. On the contrary, with the intranasal delivery we obtained a reduction in the plaque load because of a reduced pro-amyloidogenic processing of APP and a clearance of deposited Aβ by microglia. Indeed, we found that microglia are the first cellular target of hNGFp, being the only cellular type, beside BFCNs, which express TrkA in 5xFAD mice. Thus, mechanisms through which intranasal hNGFp affects APP processing does not go through BFCNs but involve a modulation of fine cytokines, including Interleukin1α and CXCL12 which we demonstrated to be upregulated in neurons after hNGFp administration as a consequence of the blockade of Tumor Necrosis Factor α (TNFα) by its soluble receptor type 2 (Capsoni et al., 2017). The data on phagocytosis of Aβ oligomers were confirmed in a parallel study performed on primary microglia cells in which it was demonstrated that NGF can increase their micropinocytosis, thus preventing the decrease in neuronal spines and the onset of deficit in long term potentiation (LTP) (Rizzi et al., 2018). LTP was also improved in the entorhinal cortex of 5xFAD mice after intranasal treatment and this correlates also with an amelioration of memory deficits (Capsoni et al., 2017).
In conclusion, a therapeutic effect able to prevent or clear Aβ deposition in the brain of the 5xFAD mouse model required a broad hNGFp biodistribution, such that could be achieved by the intranasal delivery of hNGFp, but not by the local delivery to the basal forebrain. Thus, the intranasal, but not the local, delivery of hNGFp appears to be necessary to permeate the brain with hNGFp, reach microglia which are widely distributed in the brain and provide neuroprotection and anti-neurodegenerative effects (Rizzi et al., 2018).
Efficacy of Intranasal hNGFp in Down Syndrome Mice
The fact that microglia is a target cell of NGF in the brain (Rizzi et al., 2018) and that is a primary target of intranasal hNGFp in the 5xFAD Alzheimer’s model (Capsoni et al., 2017) suggests that the microglia-mediated broad neuroprotective actions of hNGFp might be exploited in other disease states, in addition to AD. We tested this hypothesis by investigating the efficacy of hNGFp in a mouse model of Down Syndrome (DS). A progressive dementia is a common age-related clinical aspect of DS patients (Hartley et al., 2015), the neurodegeneration including the deposition of β amyloid, neurofibrillary tangles and cholinergic deficit in BFCNs (Hefti, 1986). Abnormal levels of the amyloid precursor protein APP found in Ts65Dn mice (Choi et al., 2009) lead to an impaired transport of NGF to BFCNs (Salehi et al., 2006) and the local infusion of NGF rescues the cholinergic deficit in these mice (Cooper et al., 2001). More recently, an increase of the ratio between the precursor of NGF, proNGF, and mature NGF, and imbalance in TrkA/p75NTR ratio has been found in the brain and plasma from DS patients (Iulita et al., 2014; Iulita and Cuello, 2016; Miguel et al., 2021). This imbalance is known to trigger neurodegeneration (Capsoni and Cattaneo, 2006; Fahnestock and Shekari, 2019) and to contribute to neuroinflammation (Capsoni et al., 2011; Iulita et al., 2016). Indeed, similarly to AD, an activation of astrocytes and microglia, the main mediators of inflammation, has been reported in DS subjects (Wilcock and Griffin, 2013). Given these data, therapies aimed at re-establishing the correct balance between ligands of the NGF pathway appear to have a clear rationale (Cattaneo et al., 2008; Iulita and Cuello, 2014) also for DS.
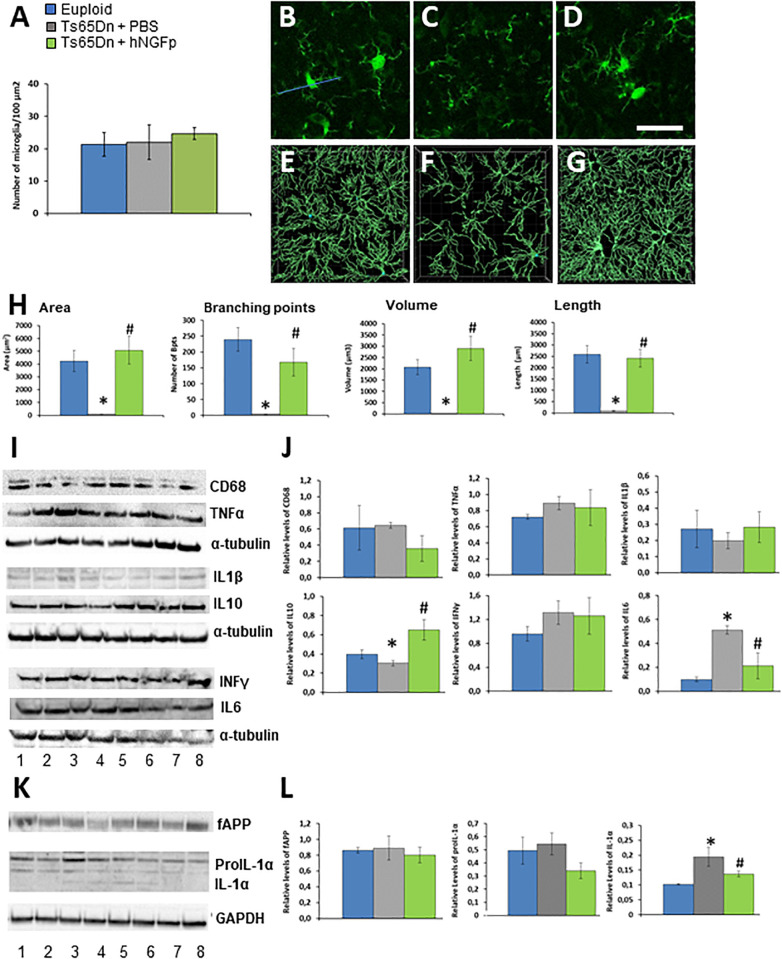
We therefore tested the effect of intranasally delivered hNGFp in Ts65Dn mice at an early stage (4 months of age), prior to overt accumulation of APP and neurodegeneration [which in this model starts at 6 months of age (Choi et al., 2009; Figures 1K,L)]. We started by investigating whether morphological alterations in microglia are found at this early stage. Microglia was reported to be dystrophic in human DS brains (Streit et al., 2009), but was never studied in TS65Dn. By single cell morphologies from confocal images, we found that, despite a similar number of microglial cells (Figure 1A) in 4 months old Ts65Dn mice microglia (identified by Iba1 immunohistochemistry) is dystrophic, with a reduction in area, volume, length and number of ramifications (Figures 1C,F,H) with respect to euploid mice (Figures 1B,E,H). The intranasal administration of hNGFp significantly restored the morphology of microglia (Figures 1D,G,H). This is highly relevant, since the morphology of microglia is directly related to its functional state.
FIGURE 1.
Intranasal hNGFp ameliorates microglial dystrophic morphology and reduces IL-1α levels in Ts65Dn mice. (A) Density of microglia cells in euploid, Ts65Dn mice treated with PBS or hNGFp. Immunohistochemistry for IBA-1 in cerebral cortex revealed morphological changes in panel (C) Ts65DN microglia with respect to panel (B) euploid mice. (D) hNGFp treatment rescues these morphological changes. Reconstruction of microglia cells by IMARIS: (E) euploid (F) Ts65Dn (G) NGFp-treated Ts65Dn mice. (H) Quantification of microglial morphological parameters. Bars are representative of mean ± SEM. *P < 0.001 vs. euploid mice, #P < 0.001 vs. Ts65Dn mice. N = 6/group. (I) Representative western blots and (J) densitometric analysis for CD68, TNFα, IL-1β, IL-10, and IL-6. (K) Representative western blots for APP and IL-1α species. Lanes 1–2 = euploid mice; 3–5 = Ts65Dn mice treated with PBS; 6–8 = Ts65Dn mice treated with hNGFp. (L) Densitometric analysis of APP, proIL-1α (graph on the left) and mature IL-1α (right panel) levels. Values have been normalized to GAPDH values. Bars are representative of mean ± SEM. *P < 0.001 vs. euploid mice, #P < 0.001 vs. Ts65Dn mice. N = 6/group. Scale bar = 10 μm.
Then we measured the levels of markers of microglia activation and cytokines. As might be expected from the fact that we analyzed brains at an age in which Alzheimer-like neurodegeneration had not yet started, we did not find a differential expression among groups for CD68, TNFα, IL-1β, and INFγ (Figures 1I,J). On the contrary, we found that hNGFp increased the expression of IL-10 while decreasing IL-6 levels (Figures 1I,J). We found that IL-1α, which we know to be decreased in Alzheimer mouse models after hNGFp treatment (Capsoni et al., 2017), was decreased. IL-1α is produced as a precursor, proIL-1α, which is cleaved to an active, lower molecular weight molecule by calpain (Kobayashi et al., 1990; Carruth et al., 1991). We observed a reduction of proIL-1α in the brain extracts from Ts65Dn mice treated with hNGFp with respect to PBS-treated mice, although not statistically significant (Figures 1K,L, P > 0.05). On the contrary, a significant reduction of mature IL-1α (Figures 1K,L) was observed after hNGFp treatment, similarly to previous findings in hNGFp-treated 5xFAD mice (Capsoni et al., 2017).
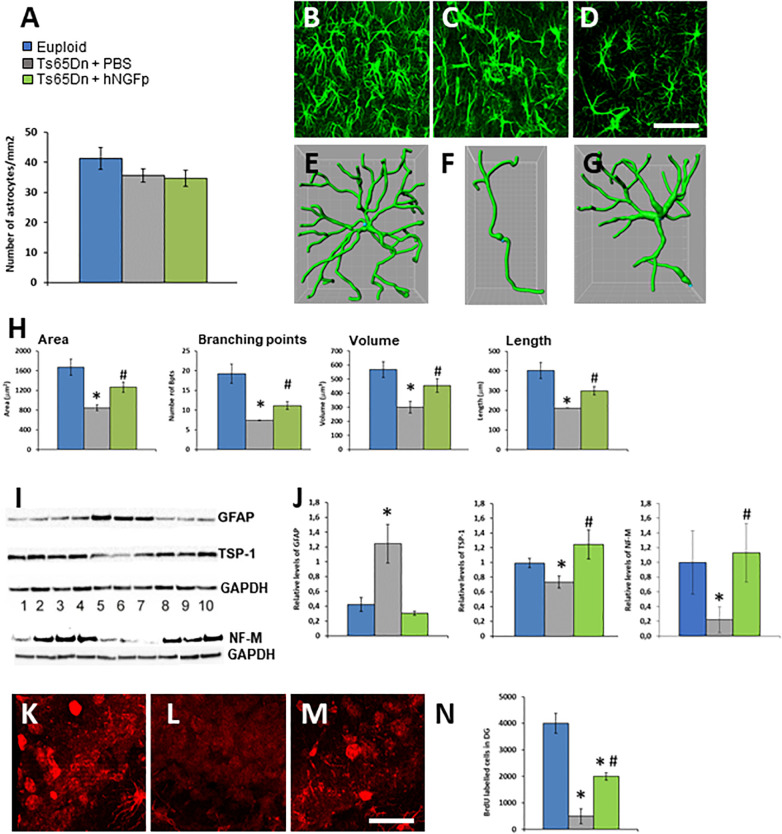
Concerning astrocytes, we found no difference in their density among the treatment groups(Figure 2A). In PBS-treated 4 months old Ts65DN mice we found a significant reduction in volume, surface area, length and number of ramifications (Figures 2C,F,H) with respect to euploid mice (Figures 2B,E,H) in the hippocampus. These changes resemble the asthenic phenotype which precedes the astrogliosis observed in mouse models of AD and in early human AD (Verkhratsky et al., 2015). The intranasal delivery of hNGFp restores the characteristic shape of astrocytes in the brain of control mice, by increasing all parameters taken into consideration (Figures 2D,G,H). The characteristic shape of astrocytes is determined also by the expression the cytoskeletal protein GFAP. We found that, despite the reduction in volume, and consistently with what reported in literature for human DS (Jorgensen et al., 1990), there is an increase of GFAP levels in Ts65Dn mice compared to control mice (Figures 2I,J). This increase is completely reverted by the intranasal administration of hNGFp (Figures 2I,J).
FIGURE 2.
Intranasal hNGFp rescues astrogliopathy and neurogenesis deficit in Ts65DN mice. (A) Density of astrocytes in euploid, Ts65Dn mice treated with PBS or hNGFp. Immunohistochemistry for GFAP in hippocampus revealed morphological changes in panel (C) Ts65DN astrocytes with respect to panel (B) euploid mice. (D) hNGFp treatment rescues these morphological changes. Reconstruction of astrocytes by IMARIS: (E) euploid (F) Ts65Dn (G) NGFp-treated Ts65Dn mice. (H) Quantification of astrocytic morphological parameters. Bars are representative of mean ± SEM. *P < 0.05 vs. euploid mice, #P < 0.05 vs. Ts65Dn mice. N = 6/group. Scale bar = 35 μm. (I) Western blot for GFAP, TPS-1 and NF-M. Lanes 1–4 = euploid mice; 5–7 = Ts65Dn mice treated with PBS; 8–10 = Ts65Dn mice treated with hNGFp. (J) Densitometric analysis of GFAP, TPS-1, and NF-M levels. Values have been normalized to GAPDH values. Adult hippocampal neurogenesis is deficient in Ts65Dn mice compared to littermates and it is partially rescued by treatment with hNGFp. (K–M) examples from panel (K) euploid, (L) Ts65Dn, and (M) Ts65Dn dentate gyrus. (N) Stereological quantification of BrdU-labeled cells. Bars are representative of mean ± SEM. *P < 0.05 vs. euploid mice, #P < 0.05 vs. Ts65Dn mice. Scale bar = 200 μm.
In DS and in Ts65Dn mice cognitive deficits have been associated to structural abnormalities in dendritic spines. A critical factor for spine development is the production of thrombospondin 1 (TPS-1) by astrocytes. Indeed, decreased levels of TPS-1 have been found in the conditioned medium of cultured DS astrocytes and hypothesized to contribute to the reduced synaptogenesis (Torres et al., 2018). We found that also in the brain of 4 months old Ts65DN mice there is a decrease in TPS-1 protein, which was reverted to normal by the intranasal administration of hNGFp (Figures 2I,J).
Astrocytic homeostatic functions in the maintenance of neurogenesis are impaired in DS (Sloan and Barres, 2014). Moreover, we recently found that proNGF/NGF imbalance determines a reduced adult neurogenesis in the hippocampus dentate gyrus (Corvaglia et al., 2019). Also, adult neurogenesis in the dentate gyrus of young Ts65Dn mice showed markedly fewer BrdU-labeled cells than euploid animals (Clark et al., 2006). Based on these data, and on the fact that the intranasal administration of hNGFp increases the production of the chemokine CXCL12 (Capsoni et al., 2017) which is a pro-neurogenesis factor (Li et al., 2012), we measured the number of BrDU-immunoreactive cells in the dentate gyrus of 5 months old TS65Dn mice. We found a dramatic reduction of neurogenesis in the dentate gyrus of TS65Dn mice (Figures 2K–N), which was partially but significantly recovered by intranasal hNGFp (Figures 2K–N). Consistent with the increased neurogenesis with hNGFp, we found that hNGFp restored the levels the neuronal marker Neurofilament-M (Figures 2I,J), which was decreased in Ts65Dn mice.
In conclusion, we found that hNGFp treatment rescues astrogliosis, dystrophic microglia and neurogenesis deficits in the brain of 4 months old TS65Dn mice.
General Conclusion and New Perspectives
From the data described in this perspective paper, we conclude that the links between deficits or alterations in the NGF system and AD go well beyond the long-established neurotrophic actions of NGF on BFCNs. Indeed, the intranasal delivery studies allowed to uncover that the cellular targets for NGF actions in the brain are more widespread than envisaged so far, including broadly distributed microglia and astrocytes. Given this finding, we conclude that intranasal hNGFp can be applied to other neurodegenerative and neurodevelopmental diseases in which cholinergic neurons are not the primary target of hNGFp action and neuroinflammation plays a relevant role. Thus, we conclude that the spectrum of neurodegenerative diseases that are amenable to be treated by hNGFp is very broad. In line with this conclusion, we presented new data demonstrating that intranasally delivered hNGFp has a potent neuroprotective action on the early phenotypic deficits in the TS65Dn mouse model of Down Syndrome and we propose that hNGFp could be used for the treatment of the progressive dementia affecting DS patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Italian Ministry of Health.
Author Contributions
SC designed the research, performed the research, and analyzed the data and wrote the manuscript. AC designed the research and wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This study was supported by the Jerome LeJeune Foundation grant to AC entitled “Toward an NGF-based therapy for Down Syndrome.”
References
- Bruno M. A., Leon W. C., Fragoso G., Mushynski W. E., Almazan G., Cuello A. C. (2009). Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer disease. J. Neuropathol. Exp. Neurol. 68 857–869. 10.1097/NEN.0b013e3181aed9e6 [DOI] [PubMed] [Google Scholar]
- Capsoni S., Cattaneo A. (2006). On the molecular basis linking Nerve Growth Factor (NGF) to Alzheimer’s disease. Cell Mol. Neurobiol. 26 619–633. 10.1007/s10571-006-9112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S., Brandi R., Arisi I., D’Onofrio M., Cattaneo A. (2011). A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer’s disease neurodegeneration in the AD11 anti-NGF mouse model. CNS Neurol. Disord. Drug Targets 10 635–647. 10.2174/187152711796235032 [DOI] [PubMed] [Google Scholar]
- Capsoni S., Covaceuszach S., Ugolini G., Spirito F., Vignone D., Stefanini B., et al. (2009). Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J. Alzheimers Dis. 16 371–388. 10.3233/JAD-2009-0953 [DOI] [PubMed] [Google Scholar]
- Capsoni S., Giannotta S., Cattaneo A. (2002). Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc. Natl. Acad. Sci. U.S.A. 99 12432–12437. 10.1073/pnas.192442999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S., Malerba F., Carucci N. M., Rizzi C., Criscuolo C., Origlia N., et al. (2017). The chemokine CXCL12 mediates the anti-amyloidogenic action of painless human nerve growth factor. Brain 140 201–217. 10.1093/brain/aww271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S., Marinelli S., Ceci M., Vignone D., Amato G., Malerba F., et al. (2012). Intranasal “painless” human Nerve Growth Factor [corrected] slows amyloid neurodegeneration and prevents memory deficits in App X PS1 mice. PLoS One 7:e37555. 10.1371/journal.pone.0037555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth L. M., Demczuk S., Mizel S. B. (1991). Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J. Biol. Chem. 266 12162–12167. 10.1016/s0021-9258(18)98874-5 [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Calissano P. (2012). Nerve growth factor and Alzheimer’s disease: new facts for an old hypothesis. Mol. Neurobiol. 46 588–604. 10.1007/s12035-012-8310-9 [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Capsoni S. (2019). Painless nerve growth factor: a TrkA biased agonist mediating a broad neuroprotection via its actions on microglia cells. Pharmacol. Res. 139 17–25. 10.1016/j.phrs.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Capsoni S., Paoletti F. (2008). Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J. Alzheimers Dis. 15 255–283. 10.3233/jad-2008-15210 [DOI] [PubMed] [Google Scholar]
- Choi J. H., Berger J. D., Mazzella M. J., Morales-Corraliza J., Cataldo A. M., Nixon R. A., et al. (2009). Age-dependent dysregulation of brain amyloid precursor protein in the Ts65Dn Down syndrome mouse model. J. Neurochem. 110 1818–1827. 10.1111/j.1471-4159.2009.06277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Schwalbe J., Stasko M. R., Yarowsky P. J., Costa A. C. (2006). Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp. Neurol. 200 256–261. 10.1016/j.expneurol.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Cooper J. D., Salehi A., Delcroix J. D., Howe C. L., Belichenko P. V., Chua-Couzens J., et al. (2001). Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc. Natl. Acad. Sci. U.S.A. 98 10439–10444. 10.1073/pnas.181219298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaglia V., Cilli D., Scopa C., Brandi R., Arisi I., Malerba F., et al. (2019). ProNGF is a cell-type-specific mitogen for adult hippocampal and for induced neural stem cells. Stem Cells 37 1223–1237. 10.1002/stem.3037 [DOI] [PubMed] [Google Scholar]
- Covaceuszach S., Capsoni S., Marinelli S., Pavone F., Ceci M., Ugolini G., et al. (2010). In vitro receptor binding properties of a “painless” NGF mutein, linked to hereditary sensory autonomic neuropathy type V. Biochem. Biophys. Res. Commun. 391 824–829. 10.1016/j.bbrc.2009.11.146 [DOI] [PubMed] [Google Scholar]
- Covaceuszach S., Capsoni S., Ugolini G., Spirito F., Vignone D., Cattaneo A. (2009). Development of a non invasive NGF-based therapy for Alzheimer’s disease. Curr. Alzheimer Res. 6 158–170. 10.2174/156720509787602870 [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Pentz R., Hall H. (2019). The brain NGF metabolic pathway in health and in Alzheimer’s pathology. Front. Neurosci. 13:62. 10.3389/fnins.2019.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa R., Garcia A. A., Braschi C., Capsoni S., Maffei L., Berardi N., et al. (2005). Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 102 3811–3816. 10.1073/pnas.0500195102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria S. V., Hanson L. R., Frey W. H., II (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharm. Sci. 99 1654–1673. 10.1002/jps.21924 [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Garcia P. D., Rutter W. J. (1988). Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J. Biol. Chem. 263 6810–6815. 10.1016/s0021-9258(18)68715-0 [DOI] [PubMed] [Google Scholar]
- Einarsdottir E., Carlsson A., Minde J., Toolanen G., Svensson O., Solders G., et al. (2004). A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum. Mol. Genet. 13 799–805. 10.1093/hmg/ddh096 [DOI] [PubMed] [Google Scholar]
- Eriksdotter Jonhagen M., Nordberg A., Amberla K., Backman L., Ebendal T., Meyerson B., et al. (1998). Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 9 246–257. 10.1159/000017069 [DOI] [PubMed] [Google Scholar]
- Fahnestock M., Shekari A. (2019). ProNGF and neurodegeneration in Alzheimer’s disease. Front Neurosci. 13:129. 10.3389/fnins.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M., Michalski B., Xu B., Coughlin M. D. (2001). The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell Neurosci. 18 210–220. 10.1006/mcne.2001.1016 [DOI] [PubMed] [Google Scholar]
- Fahnestock M., Yu G., Michalski B., Mathew S., Colquhoun A., Ross G. M., et al. (2004). The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J. Neurochem. 89 581–592. 10.1111/j.1471-4159.2004.02360.x [DOI] [PubMed] [Google Scholar]
- Fasulo L., Brandi R., Arisi I., La Regina F., Berretta N., Capsoni S., et al. (2017). ProNGF drives localized and cell selective parvalbumin interneuron and perineuronal net depletion in the dentate gyrus of transgenic mice. Front. Mol. Neurosci. 10:20. 10.3389/fnmol.2017.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Wictorin K., Bjorklund A., Williams L. R., Varon S., Gage F. H. (1987). Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329 65–68. 10.1038/329065a0 [DOI] [PubMed] [Google Scholar]
- Francke U., de Martinville B., Coussens L., Ullrich A. (1983). The human gene for the beta subunit of nerve growth factor is located on the proximal short arm of chromosome 1. Science 222 1248–1251. 10.1126/science.6648531 [DOI] [PubMed] [Google Scholar]
- Frey W., Liu J., Chen X., Thorne R. G., Fawcett J. R., Ala T. A., et al. (1997). Delivery of 125I-NGF to the brain via the olfactory route. Drug Delivery 42:6. [Google Scholar]
- Gascon S., Jann J., Langlois-Blais C., Plourde M., Lavoie C., Faucheux N. (2021). Peptides derived from growth factors to treat Alzheimer’s disease. Int. J. Mol. Sci. 22:6071. 10.3390/ijms22116071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E. (2006). “Cholinesterase in human brain: the effect of cholinesterase inhibitors on Alzheimer’s disease and related disorders,” in The Brain Cholinergic System in Health and Disease, eds Giacobini E., Pepeu G. (London: Informa Healthcare; ), 235–264. 10.1201/b14486-19 [DOI] [Google Scholar]
- Gnahn H., Hefti F., Heumann R., Schwab M. E., Thoenen H. (1983). NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 285 45–52. 10.1016/0165-3806(83)90107-4 [DOI] [PubMed] [Google Scholar]
- Hartley D., Blumenthal T., Carrillo M., DiPaolo G., Esralew L., Gardiner K., et al. (2015). Down syndrome and Alzheimer’s disease: common pathways, common goals. Alzheimers Dement. 11 700–709. 10.1016/j.jalz.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F. (1986). Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 6 2155–2162. 10.1523/JNEUROSCI.06-08-02155.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M., Li Y., Parada L. F., Kinsman S., Chen C. K., Valletta J. S., et al. (1992). p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron 9 465–478. 10.1016/0896-6273(92)90184-f [DOI] [PubMed] [Google Scholar]
- Iulita M. F., Cuello A. C. (2014). Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 35 338–348. 10.1016/j.tips.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Iulita M. F., Cuello A. C. (2016). The NGF metabolic pathway in the CNS and its dysregulation in down syndrome and Alzheimer’s disease. Curr. Alzheimer Res. 13 53–67. 10.2174/1567205012666150921100030 [DOI] [PubMed] [Google Scholar]
- Iulita M. F., Caraci F., Cuello A. C. A. (2016). Link between nerve growth factor metabolic deregulation and amyloid-beta-driven inflammation in down syndrome. CNS Neurol. Disord. Drug Targets 15 434–447. 10.2174/1871527315666160321104916 [DOI] [PubMed] [Google Scholar]
- Iulita M. F., Do Carmo S., Ower A. K., Fortress A. M., Flores Aguilar L., Hanna M., et al. (2014). Nerve growth factor metabolic dysfunction in Down’s syndrome brains. Brain 137(Pt 3), 860–872. 10.1093/brain/awt372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen O. S., Brooksbank B. W., Balazs R. (1990). Neuronal plasticity and astrocytic reaction in Down syndrome and Alzheimer disease. J. Neurol. Sci. 98 63–79. 10.1016/0022-510x(90)90182-m [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto K., Saido T., Kawasaki H., Oppenheim J. J., Matsushima K. (1990). Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc. Natl. Acad. Sci. U.S.A. 87 5548–5552. 10.1073/pnas.87.14.5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. (1987). Nerve growth factor treatment after brain injury prevents neuronal death. Science 235 214–216. 10.1126/science.3798108 [DOI] [PubMed] [Google Scholar]
- Lee R., Kermani P., Teng K. K., Hempstead B. L. (2001). Regulation of cell survival by secreted proneurotrophins. Science 294 1945–1948. 10.1126/science.1065057 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. (1952). Effects of mouse tumor transplantation on the nervous system. Ann. N. Y. Acad. Sci. 55 330–344. 10.1111/j.1749-6632.1952.tb26548.x [DOI] [PubMed] [Google Scholar]
- Li M., Hale J. S., Rich J. N., Ransohoff R. M., Lathia J. D. (2012). Chemokine CXCL12 in neurodegenerative diseases: an SOS signal for stem cell-based repair. Trends Neurosci. 35 619–628. 10.1016/j.tins.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O., Wahlberg L. U. (2008). Encapsulated cell biodelivery of GDNF: a novel clinical strategy for neuroprotection and neuroregeneration in Parkinson’s disease? Exp. Neurol. 209 82–88. 10.1016/j.expneurol.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Malerba F., Paoletti F., Bruni Ercole B., Materazzi S., Nassini R., Coppi E., et al. (2015). Functional characterization of human ProNGF and NGF mutants: identification of NGF P61SR100E as a “Painless” lead investigational candidate for therapeutic applications. PLoS One 10:e0136425. 10.1371/journal.pone.0136425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba F., Paoletti F., Capsoni S., Cattaneo A. (2011). Intranasal delivery of therapeutic proteins for neurological diseases. Expert Opin. Drug Deliv. 8 1277–1296. 10.1517/17425247.2011.588204 [DOI] [PubMed] [Google Scholar]
- Miguel J. C., Perez S. E., Malek-Ahmadi M., Mufson E. J. (2021). Cerebellar calcium-binding protein and neurotrophin receptor defects in down syndrome and Alzheimer’s disease. Front. Aging Neurosci. 13:645334. 10.3389/fnagi.2021.645334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Behbahani H., Eriksdotter M. (2019). Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Front. Neurosci. 13:38. 10.3389/fnins.2019.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley W. C., Rutkowski J. L., Tennekoon G. I., Gemski J., Buchanan K., Johnston M. V. (1986). Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 387 53–62. 10.1016/0169-328x(86)90020-3 [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Conner J. M., Kordower J. H. (1995). Nerve growth factor in Alzheimer’s disease: defective retrograde transport to nucleus basalis. Neuroreport 6 1063–1066. 10.1097/00001756-199505090-00028 [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Counts S. E., Ginsberg S. D., Mahady L., Perez S. E., Massa S. M., et al. (2019). Nerve growth factor pathobiology during the progression of Alzheimer’s disease. Front. Neurosci. 13:533. 10.3389/fnins.2019.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediaditakis I., Kourgiantaki A., Prousis K. C., Potamitis C., Xanthopoulos K. P., Zervou M., et al. (2016b). BNN27, a 17-spiroepoxy steroid derivative, interacts with and activates p75 neurotrophin receptor, rescuing cerebellar granule neurons from apoptosis. Front. Pharmacol. 7:512. 10.3389/fphar.2016.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediaditakis I., Efstathopoulos P., Prousis K. C., Zervou M., Arevalo J. C., Alexaki V. I., et al. (2016a). Selective and differential interactions of BNN27, a novel C17-spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation. Neuropharmacology 111 266–282. 10.1016/j.neuropharm.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Podlesniy P., Kichev A., Pedraza C., Saurat J., Encinas M., Perez B., et al. (2006). Pro-NGF from Alzheimer’s disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosis. Am. J. Pathol. 169 119–131. 10.2353/ajpath.2006.050787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii M. S., Baumann T. L., Bakay R. A., Ostrove J. M., Siffert J., Fleisher A. S., et al. (2014). A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimers Dement. 10 571–581. 10.1016/j.jalz.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Rafii M. S., Tuszynski M. H., Thomas R. G., Barba D., Brewer J. B., Rissman R. A., et al. (2018). Adeno-associated viral vector (Serotype 2)-Nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol. 75 834–841. 10.1001/jamaneurol.2018.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi C., Tiberi A., Giustizieri M., Marrone M. C., Gobbo F., Carucci N. M., et al. (2018). NGF steers microglia toward a neuroprotective phenotype. Glia 66 1395–1416. 10.1002/glia.23312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti F., Capsoni S., Comparini A., Di Daniel E., Franzot J., Gonfloni S., et al. (2000). Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 20 2589–2601. 10.1523/JNEUROSCI.20-07-02589.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A., Delcroix J. D., Belichenko P. V., Zhan K., Wu C., Valletta J. S., et al. (2006). Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51 29–42. 10.1016/j.neuron.2006.05.022 [DOI] [PubMed] [Google Scholar]
- Seiler M., Schwab M. E. (1984). Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 300 33–39. 10.1016/0006-8993(84)91338-6 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2000). The genetics and molecular pathology of Alzheimer’s disease: roles of amyloid and the presenilins. Neurol. Clin. 18 903–922. 10.1016/s0733-8619(05)70232-2 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2001). Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81 741–766. 10.1152/physrev.2001.81.2.741 [DOI] [PubMed] [Google Scholar]
- Sloan S. A., Barres B. A. (2014). Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 27 75–81. 10.1016/j.conb.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Braak H., Xue Q. S., Bechmann I. (2009). Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 118 475–485. 10.1007/s00401-009-0556-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron C., Fasulo L., Capsoni S., Malerba F., Marinelli S., Paoletti F., et al. (2013). ProNGF\NGF imbalance triggers learning and memory deficits, neurodegeneration and spontaneous epileptic-like discharges in transgenic mice. Cell Death Differ. 20 1017–1030. 10.1038/cdd.2013.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M. D., Garcia O., Tang C., Busciglio J. (2018). Dendritic spine pathology and thrombospondin-1 deficits in Down syndrome. Free Radic. Biol. Med. 114 10–14. 10.1016/j.freeradbiomed.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski M. H., Thal L., Pay M., Salmon D. P., U H. S., Bakay R., et al. (2005). A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 11 551–555. 10.1038/nm1239 [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H., Yang J. H., Barba D., U H. S., Bakay R. A., Pay M. M., et al. (2015). Nerve Growth factor gene therapy: activation of neuronal responses in alzheimer disease. JAMA Neurol. 72 1139–1147. 10.1001/jamaneurol.2015.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Marutle A., Rodriguez-Arellano J. J., Nordberg A. (2015). Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer’s disease. Neuroscientist 21 552–568. 10.1177/1073858414547132 [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. (1982). Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215 1237–1239. 10.1126/science.7058341 [DOI] [PubMed] [Google Scholar]
- Wilcock D. M., Griffin W. S. (2013). Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J. Neuroinflamm. 10:84. 10.1186/1742-2094-10-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., et al. (1986). Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc. Natl. Acad. Sci. U.S.A. 83 9231–9235. 10.1073/pnas.83.23.9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Knowles J. K., Lu Q., Zhang H., Arancio O., Moore L. A., et al. (2008). Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS One 3:e3604. 10.1371/journal.pone.0003604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Tran K. C., Zeng A. Y., Massa S. M., Longo F. M. (2020). Small molecule modulation of the p75 neurotrophin receptor inhibits multiple amyloid beta-induced tau pathologies. Sci. Rep. 10:20322. 10.1038/s41598-020-77210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.