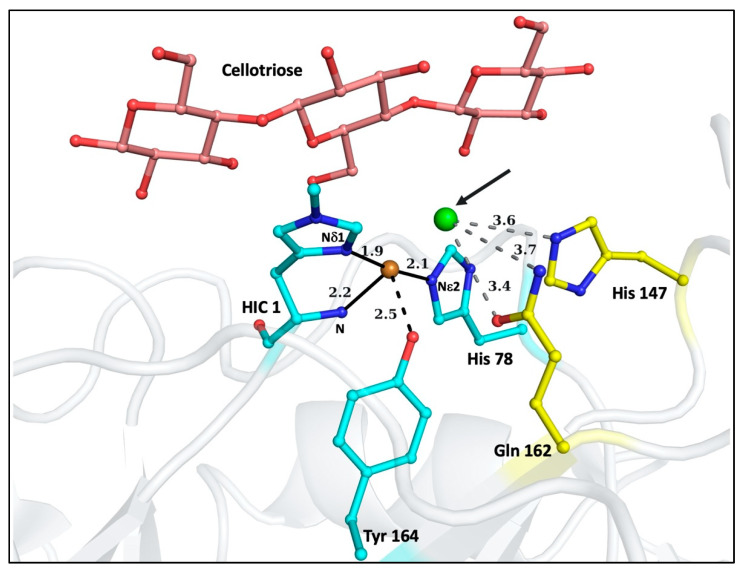
Figure 1.
Structure of Lentinus similis AA9A (LsAA9A), PDB ID: 5ACF showing its active site with the bound oligosaccharide cellotriose. The 1st coordination sphere of the copper (orange sphere) is formed by the residues His1, His78, and Tyr 164 (colored in cyan). The coordination distances are shown in Å. As the oligosaccharide is bound, the axial water is displaced in this structure, but is visible in Figure 4. At the equatorial position, chloride (green sphere) is bound instead of water. This position (pointed with a black arrow) is the presumed binding site for the activated oxygen species. The 2nd coordination sphere residues are His147 and Gln162 (colored in yellow). His147 is conserved across all AA9 LPMOs. The distances from His147 and Gln162 to the chloride in Å are shown in grey. The residues are represented in ball and stick representation. The figure was prepared in Pymol.