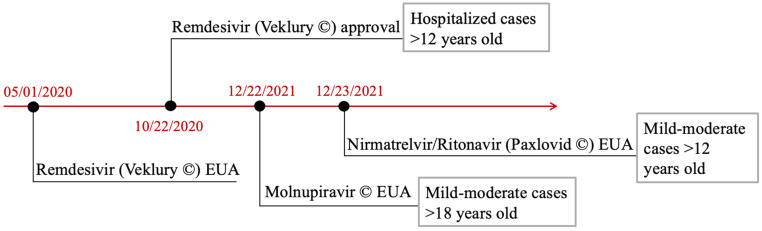
Figure 1.
FDA timeline of antivirals approval and EUAs. Veklury® EUA was formalized in January 2020. Its definitive approval occurred in October 2020. Molnupinavir and Paxlovid® EUAs followed in December 2021. Indications are also presented, in gray-outlined boxes, on the side of each authorized therapeutic agent. Abbreviations. FDA: Food and Drug Administration; EUA: Emergency Use Authorization.